A Multicenter, Retrospective Evaluation of The Efficacy of a Novel Posterior Sacroiliac Joint Fusion System: Initial Case Series
Robert E. Tibbs Jr., MD1*, Ankur Khosla, MD, MBA, MS2, Jonathan Grossman, MD3, Joseph Savino, MD4
1Oklahoma Spine Hospital, 14100 Parkway Commons Dr Suite 100, Oklahoma City, OK 73134.
2AK Pain & Spine Center 10020 Research Forest Drive Unit Magnolia, TX 77354.
3Saint Agnes Care Pain & Spine, 1510 E Herndon Ave. Ste 230 Fresno, CA 93720.
4Pain Specialists of Southern Oregon 825 Bennett Ave, Medford, OR 97504.
*Corresponding Author: Robert E. Tibbs Jr. Oklahoma Spine Hospital, 14100 Parkway Commons Dr Suite 100, Oklahoma City, OK 73134, USA.
Received: 22 July 2025; Accepted: 29 July 2025; Published: 03 October 2025
Article Information
Citation: Robert E. Tibbs Jr., Ankur Khosla, Jonathan Grossman, Joseph Savino. A Multicenter, Retrospective Evaluation of Patient Reported Outcomes for a Novel Posterior Sacroiliac Joint Fusion System in an Initial Case Series. Journal of Spine Research and Surgery. 7 (2025): 98-104.
Share at FacebookAbstract
Background:
The advent of minimally invasive techniques has significantly advanced sacroiliac joint (SIJ) fusion procedures. Previous studies have reported improvements in pain and functional improvement using Visual Analog Scale (VAS) and the Oswestry Disability Index (ODI) following SIJ fusion. Clinical outcomes have been modest, with some patients not achieving the Minimal Clinically Important Difference (MCID) in pain and functional improvement with respect to certain techniques and approaches. The TiLink-P®, SIJ fusion implant is a novel 3D-printed titanium implant with an advanced surface technology designed to improve patient outcomes in SIJ fusion via a posterior approach. This study aimed to assess the efficacy of the TiLink-P® Minimally Invasive SIJ fusion procedure and implant in a case series.
Methods:
A multicenter retrospective analysis included four US sites. Patients who were previously treated with TiLink-P® between October 1, 2023 to January 4, 2024, were consecutively screened for eligibility. De-identified demographic data, preoperative pain, disability scores, and postoperative outcomes at approximately one year were collected. The primary outcome measures were changes in pain using NRS (Numeric Rating Scale) or VAS and disability using ODI from preoperative to approximately one-year postoperative.
Results:
A total of 21 subjects were included for analysis with a mean age of 66.6 years, and a mean BMI of 32.1 . The mean follow-up period was 11.6 months. Compared to the preoperative values, the follow up back pain score (VAS) and ODI values improved 60.3%, from 83.3 to 31.1 (p < 0.0001) and 71.9% from 52.8 to 8.3 (p = 0.001) respectively.
Clinical Relevance:
There were no device or procedure events during the study period. Pain and ODI scores were statistically improved at approximately 1 year follow-up.
Conclusions/Level of Evidence:
The TiLink-P® posterior SIJ fusion system demonstrated significant pain reduction and functional improvement scores without procedure or device-related adverse events.
Keywords
Transfix, Nano surface technology, 3D printed titanium, minimally invasive surgery, sacroiliac joint (SIJ) fusion, compression
Transfix articles, Nano surface technology articles, 3D printed titanium articles, minimally invasive surgery articles, sacroiliac joint (SIJ) fusion articles, compression articles
Article Details
Introduction
Low back pain represents a significant financial burden in the United States [1]. Recent research highlights the sacroiliac joint (SIJ) as a possible pain generator, with estimates between 15% to 30% of low back pain attributed to the SIJ [2,3]. Traditional management of SIJ pain begins with conservative management. Patients who do not experience improvement are then referred to interventional therapies, such as steroid injections and lateral branch ablation. Surgery is considered when a patient has failed conservative care after six months [4, 5].
While open SIJ fusion procedures have been performed since the 1920s, advancements in minimally invasive techniques have emerged to minimize the recovery period [6]. These minimally invasive approaches have gained widespread adoption by focusing on improving pain and disability/functional improvement scores, including the Visual Analog Scale (VAS) and the Oswestry Disability Index (ODI) [4, 7, 8]. Previous studies have evaluated the impact of fusion on patient reported outcomes (PROs) across various approaches and implant materials and designs [9].
Martin et al. summarized a substantial body of literature indicating improvements in disability and pain as measured by ODI, VAS, and Numeric Pain Scale (NRS) using a lateral trans iliac approach [9, 10-12]. Despite these notable improvements in PROs, the overall observed clinical improvement was modest [11]. A prospective, randomized controlled trial of 141 subjects who underwent lateral SIJ fusion were evaluated at 6 and 24 months following surgery. The authors reported that approximately 30% of subjects did not meet the minimum clinically important difference (MCID) for VAS, and 27% did not meet MCID for ODI [13]. Similarly, in a single arm prospective study of 172 subjects, 16% failed to meet MCID for VAS, and 34% of subjects did not meet MCID for ODI [14]. Both studies defined MCID as a change of greater than 20 points for VAS, and greater than a 15 point change for ODI.
Data supports that the lateral approach in an SIJ fusion procedure presents significant safety risks [14, 15]. Specifically, Polly et al. reported that 22 out of 102 subjects experienced device or procedure related adverse events (21.6%) [15]. A systematic review of 14 studies including 720 subjects reported a procedure-related complication rate of 11.1%, with surgical wound infection being the most common adverse event, and a 2.6% revision rate. It should be recognized that the specific complication profile of the procedure had not been thoroughly characterized [14].
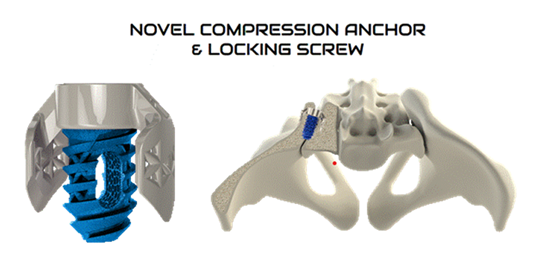
The current study involves the TiLink-P® posterior implant (Figure 1).
This novel and innovative 3D printed titanium posterior SIJ fusion standalone implant is designed with an open trellis (lattice) structure, a transfixing compression anchor, and Nanotex® (Bloomfield Hills, MI) surface technology. The implant is designed to transfix the ilium and sacrum while simultaneously compressing the sacroiliac joint to enhance stability and promote improved functional outcomes.
The aim of this prospective study was to ascertain the effectiveness of the TiLink-P® SIJ Fusion System at approximately one-year post-surgery in a case series using established PROs as a measure.
Materials and Methods:
This was a multi-center, retrospective study evaluating the performance of SIJ fusion using TiLink-P®. This study included four US sites, with patients who were treated with TiLink-P® from October 1, 2023, through January 4, 2024, and screened consecutively for eligibility. This time frame marks the initial experience with the device following FDA clearance on September 25, 2023. The study inclusion and exclusion criteria are shown in Table 1.
Table 1: Study Eligibility: Inclusion-Exclusion criteria.
|
Inclusion Criteria |
||
|
1. |
Previously treated on-label with standalone unilateral or bilateral TiLink-P® |
|
|
2. |
Diagnosed with SIJ pain or degenerative sacroiliitis |
|
|
3. |
At least 21 years of age at the time of surgery |
|
|
4. |
Have completed baseline and 12-month postoperative patient reported outcomes |
|
|
Exclusion Criteria |
||
|
1. |
Index surgery is a revision of a previous sacroiliac fusion |
|
|
2. |
Diagnosed with other known sacroiliac pathology such as: |
|
|
• |
Sacral dysplasia |
|
|
• |
Inflammatory sacroiliitis (e.g. ankylosing spondylitis or other HLA- |
|
|
associated spondyloarthropathy |
||
|
• |
Tumor |
|
|
• |
Infection |
|
|
• |
Acute fracture |
|
|
• |
Crystal arthropathy |
|
|
3. |
History of recent (<1 year) major trauma to pelvis prior to index surgery |
|
|
4. |
Previously diagnosed osteoporosis (defined as prior T-score < -2.5 or history of |
|
|
osteoporotic fracture) or prior use of drug therapy for osteoporosis |
||
|
5. |
Known allergy to titanium or titanium alloys |
|
|
6. |
Patient was a prisoner or a ward of the state at the time of surgery |
|
|
7. |
Patient was pregnant at the time of surgery |
|
Table 1: Study Eligibility: Inclusion-Exclusion criteria.
Data were collected for all subjects meeting the study inclusion criteria. Baseline demographics, medical history, hospital utilization, and postoperative outcomes, including the incidence of adverse events, were systematically recorded and analyzed. Due to varying patient availability for follow-up across multiple sites, the one-year follow-up period was found to be a mean of (11.6 ± 1.7) months post-surgery and will be termed “approximately 1 year follow up”. Patient reported outcomes (PROs) were collected at preoperative baseline and at approximately one-year postoperative. A one-sample t-test was conducted to test the null hypothesis that the mean follow-up duration did not differ from 12 months. The analysis confirmed that reporting the postoperative observation period as “approximately 1-year follow-up” was appropriate (Prism v10, GraphPad Software, San Diego, CA).
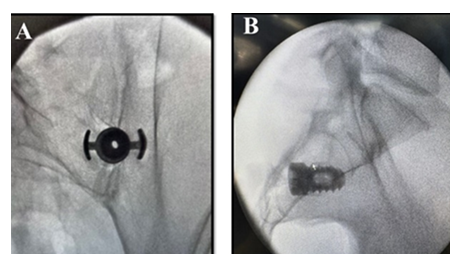
Patients were placed in prone position on a radiolucent table under general anesthesia or monitored anesthesia care (MAC). Multiaccess fluoroscopic guidance, including inlet oblique, outlet oblique, and lateral views were used. The SIJ was identified using the inlet oblique view. The entry point was identified below the inferior aspect of the posterior superior iliac spine and then an incision was made. Surgeons subsequently advance the guide pin into the joint, confirming trajectory with outlet oblique and lateral fluoroscopic views. A soft tissue dilator was placed over the guide pin down to the posterior margin of the sacrum using a lateral view. The drill guide was advanced over the dilator until firmly docked over the medial aspect of the ilium and the lateral aspect of the sacrum. The long tangs into the SIJ were confirmed with lateral, outlet oblique, and a gun barrel view. The soft tissue dilator and guide pin were removed. The drill was advanced through the drill guide to a positive stop thereby decorticating the joint while using outlet oblique and lateral views to confirm proper depth and trajectory. The drill was removed, and the implant inserter was advanced with the anchor wings down to a positive stop. At this point surgeons confirm the anchor transfixed the SIJ with outlet oblique and lateral views (Figure 2).
A hexalobe driver was used to deploy the screw to fixate and hold the anchor in place. Finally, the implant inserter was removed, and the joint was post packed with approximately 5cc of bone graft biologic material behind the TiLink-P® SIJ fusion implant.
The primary objective was to assess the effectiveness of the study device as measured by pain and disability scores using PROs including NRS as it relates to SIJ pain, and ODI. PROs were collected from preoperative visits and at approximately one year postoperatively. For NRS, patients rated their pain on a numerical rating scale from 0 - 10. Due to varied pain scores reported across different clinics, pain scores were rescaled to a standardized Visual Analogue Scale (VAS) from 0 - 100-point score for analysis. This method has been evaluated and justified in previous published research [16, 17]. For VAS, patients marked their pain on a 0 - 100 mm line. In either case 0 indicates no pain and 10 or 100 signifies the worst pain conceivable. Disability scores were calculated based on patients’ responses to the ODI questionnaire. A score of 0 indicates no disability and 100 indicates complete disability.
Additionally, MCID and substantial clinical benefit (SCB) were evaluated. MCID was defined as a difference of 20 points for pain (on the 0 – 100 scale) and 15 points for ODI [18, 19]. SCB thresholds for pain was defined as a 25-point difference or an improvement of at least 41.4%. The SCB threshold for ODI was established as an 18.8-point difference or improvement of at least 36.8% [20, 21]. Furthermore, the percentage of patients with at least 20% improvement in pain and function were also calculated.
The secondary objective was to assess the safety of the TiLink-P® system by reporting the incidence of procedure or device-related Serious Adverse Events (SAE) and Secondary Surgical Interventions (SSI) at the index surgery site within the follow-up period. Reported SAEs would be adjudicated by an independent reviewer blinded to the clinical care and outcomes of the subjects.
Statistical analyses included descriptive statistics reported as mean and standard deviations for numerical variables and counts and percentages for categorical variables. Pre-to-postoperative differences were assessed by Wilcoxon matched-pairs sign ranked test. Post-hoc power analyses were performed to determine the power (1 - β) achieved to detect significant differences in the respective PROs from preoperative to respective follow-up. Missing data was not imputed. All statistical tests were performed using Statistical Package for the Social Sciences (SPSS, IBM, v28, Armonk, NY). It should be noted that subjects who underwent a second SIJ fusion surgery on the contralateral side of the index procedure within their one-year postoperative time window were excluded from the analysis due to possible pain and disability symptoms that could not be differentiated between the two surgeries. Data collection, verification, and aggregation were coordinated by an independent thirdparty contract research organization under confidentiality agreement. The CRO operated independently from the product manufacturer and had no role in patient care, clinical decision-making, or data interpretation. All data were de-identified before analysis and reviewed in collaboration with the participating investigators to ensure accuracy and objectivity.
Results:
|
Parameter |
Result |
|
|
Age (years), mean (SD) |
66.6 (10.1) |
|
|
BMI, mean (SD) |
32.1 (8.0) |
|
|
Sex, N (%) |
||
|
Male |
4 (19.0%) |
|
|
Female |
17 (81.0%) |
|
|
Smoking Status, |
N (%) |
|
|
Never |
13 (61.9%) |
|
|
Previous |
3 (14.3%) |
|
|
Current |
1 (4.8%) |
|
|
Unknown |
4 (19.0%) |
|
|
Facility Type, |
N (%) |
|
|
Outpatient Hospital |
4 (19.0%) |
|
|
Ambulatory Surgery Center |
17 (81.0%) |
|
|
SI Joint, |
N (%) |
|
|
Left |
12 (57.1%) |
|
|
Right |
9 (42.9%) |
|
|
EBL (ml), mean (SD) |
6.2 (6.8) |
|
|
LOS (days), mean (SD) |
0.1 (0.3) |
|
Table 2: Demographic and surgical data.
A total of 24 subjects met the study entrance criteria. However, three subjects were excluded due to having a secondary SIJ fusion on the contralateral side within the follow-up period. As a result, 21 subjects were included in the analysis. The remaining 21 subjects consisted of 17 females and 4 males. The mean subject age was (66.6 ± 10.1) years and mean BMI was (32.1 ± 8.0). Most subjects were never smokers (61.9%), while 33.3% were either former smokers or had an unknown smoking status. The mean follow-up time was (11.6 ± 1.7) months. The 1 sample t-test comparing the study follow-up period to 12 months was not statistically significant (P > 0.25). Hence a 1-year term definition for follow-up is acceptable. Demographic and surgical data are reported in Table 2.
Most surgeries (81.0%) in this study were performed at an ambulatory surgery center (ASC), with the remaining 19% of subjects treated at an outpatient hospital. No patients required an inpatient stay. Nine subjects (42.9%) had their right SI joint treated, and 12 subjects (57.1%) had their left SI joint treated. The estimated blood loss was (6.2 ± 6.8) ml. All subjects were discharged on the same day, except for one subject who required an overnight stay.
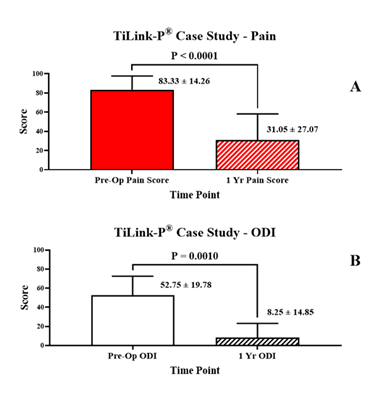
Patient reported outcomes are reported in Table 3 and displayed graphically in Figure 3
|
Patient Reported Outcome |
|||||
|
N |
Pre-Operative |
Post-Operative |
P Value |
||
|
VAS Pain, mean (SD) |
21 |
83.3 (14.3) |
31.1 (27.1) |
<0.0001 |
|
|
ODI, mean (SD) |
12 |
52.8 (19.8) |
8.3 (14.9) |
0.001 |
|
Table 3: Patient reported outcomes illustrating statistically significant decreases at 1 year follow up compared to pre-operative levels. VAS Pain scores decreased (P < 0.0001). ODI decreased (P = 0.001).
Respective improvements in scores are shown in Table 4.
|
Patient Reported Outcome Improvement |
|||||
|
MCID |
SCB |
||||
|
N |
Δ |
% Improvement |
N (%) |
N (%) |
|
|
VAS Pain, mean (SD) |
21 |
52.3 (32.3) |
60.3 (35.6) |
18 (85.7%) |
16 (76.2%) |
|
ODI, mean (SD) |
12 |
44.5 (24.7) |
71.9 (58.6) |
11 (91.7%) |
11 (91.7%) |
Table 4: Improvement in Patient Report Outcomes (PROs) for VAS Pain and ODI Disability measures.
The mean preoperative back pain score, VAS, was (83.3 ± 14.3), which significantly improved to (31.1 ± 27.1) at the approximate one-year postoperative time point (P < 0.0001). The mean improvement was (52.3 ± 32.3) points, representing a (60.3 ± 35.6)% improvement from preoperative pain levels. Eighteen of the 21 subjects (85.7%) achieved MCID, and 16 (76.2%) achieved SCB for back pain improvement. Twelve of the 21 subjects also had completed pre-and postoperative ODI for analysis, which also demonstrated significant improvement. The mean preoperative ODI score was (52.8 ± 19.8) and significantly improved to (8.3 ± 14.8) at the approximate one-year postoperative time point (P = 0.001). The mean ODI improvement was (44.5 ± 24.7) points, representing a (71.9 ± 58.6)% improvement in disability from preoperative evaluation. All but one of the subjects with ODI data available (91.7%) achieved both MCID and SCB for disability improvement. In addition, 85.7% of the subjects reported a VAS pain scale improvement greater than 20% while 91.7% of the subjects manifested an ODI functional improvement greater than 20%.
No serious adverse events (SAEs) related to the device or procedure, and no secondary surgeries occurred during the study period. To evaluate the strength of the study results, post-hoc power analyses were performed. These analyses demonstrated a statistical power greater than 90% (1 - β > 0.9) for both Visual Analog Scale (VAS) pain scores and Oswestry Disability Index (ODI) outcomes, indicating a very high likelihood that the study was adequately powered to detect meaningful differences in these clinical measures.
Discussion:
This study evaluated SIJ fusion from a posterior approach and demonstrated improved outcomes over those reported with minimally invasive lateral approaches. PROs from this case series demonstrated a high percentage of subjects achieving MCID and SCB improvements in pain and disability. These improvements exceed the rates reported in earlier studies, which ranged from 66% to 85% [13, 14, 18]. Additionally, the mean improvement in ODI was increased in the current study using a transfixation method compared with a previous report of distraction arthrodesis. More specifically, the current series demonstrated a (44.5± 24.7) point improvement compared with a 7.1-point improvement at one-year postoperative as reported by Endres et al [22]. A previous meta-analysis reported a higher mean ODI improvement of 25.9 points in lateral approach SIJ fusions, but still lower than the improvement observed in this study which demonstrated an ODI improvement of (71.9 ± 58.6), (P < 0.03, 1 sample t-test). The same meta-analysis reviewed 57 cohorts totaling 2,851 patients and reported an average pain improvement of 4.8 points on a 0 - 10 scale. This is similar to the observed improvement of (52.3 ± 32.3)%, (P > 0.5, 1 sample t test) on a 0 - 100 scale in the current study [23]. These results show incremental substantial gain versus previously reported outcomes in lateral SIJ approaches, supporting the use of the TiLink-P® posterior SIJ fusion system [9]. The current study results also suggest that the TiLink-P® SIJ fusion system is an effective alternative solution to the lateral approach given low reported blood loss, zero serious adverse events, and no adverse events. The study results report an improvement over previously published literature on the lateral approach to SIJ fusion [15]. Shamrock et al. performed a meta-analysis and reported an overall complication rate of 11.1% across 14 studies including 720 patients. The most frequently observed adverse events were surgical wound infection or drainage (2.1%), trochanteric bursitis (1.3%), and hematoma formation (1.1%). The revision rate was 2.6%. Device-related events occurred in 3.1% of cases, with nerve root impingement being the most common occurrence [14]. In addition, particularly with the placement of implants from the lateral approach, there have been reports of blood loss and/or hematoma formation due to injury of the superior gluteal artery [24, 25]. None of these adverse events were observed in the current study. Finally, length of stay was also substantially decreased in the current study as compared to lateral SIJ fusion. Polly et al. reported 55.9% of patients had a 1–2-day hospital stay and 2.9% were hospitalized for three days, whereas in this study, all but one patient was discharged the same day [15].
Limitations of this study include its retrospective design. However, all subjects were consecutively screened for eligibility to minimize bias. While the study involves a small sample size, a post-hoc power analysis confirmed that it achieved well above the gold standard of 80% power to detect clinically meaningful improvements in both pain and disability measures. Calculation of Cohen’s d to determine relevant effect size resulted in values of 2.41 and 2.54 respectively for Pain and ODI. The threshold for a large effect and therefore mitigation for a small sample size is only 0.8 [26]. More specifically, the effects difference is so large that a small sample size can suffice to detect a statistically significant difference with sufficient power. An additional limitation is the lack of a corresponding SIJ fusion control group. Additionally, being a multi-center study with a diverse demographic representation, the results may be more generalizable and reproducible.
Conclusions:
The findings of this study support the efficacy of the TiLink-P® sacroiliac joint fusion system for the treatment of sacroiliac joint dysfunction, including sacroiliac joint disruptions and degenerative sacroiliitis. TiLink-P® is a 3D printed titanium implant with Nanotex® surface technology, specifically engineered to transfix and compress the sacroiliac joint through a posterior approach. Patient reported outcome data demonstrated both statistically significant functional improvement and clinically meaningful reductions in pain and disability. Favorable safety findings, including low intraoperative blood loss and the absence of device or procedure related serious adverse events, further underscore the clinical utility of this system as a treatment option for sacroiliac joint fusion.
Conflicts of Interest:
This study was supported by research funding from SurGenTec, LLC. Participating investigators received fair-market-value compensation for time and effort related to data collection and reporting. The authors acknowledge the contributions of an independent third-party contract research organization that provided coordination of data management and statistical analysis under confidentiality agreement. The CRO functioned independently from the product manufacturer, which had no involvement in patient enrollment, data handling, or outcome assessment.
Robert E. Tibbs Jr., MD
Received Funding for Study: (Yes)
Conflicts of interest and financial disclosures relevant to the manuscript topic:
Received compensation as paid research from SurGenTec, LLC
Ankur Khosla, MD, MBA, MS
Received Funding for Study: (Yes)
Conflicts of interest and financial disclosures relevant to the manuscript topic:
Institutional funding received through (Square Spine Ventures LLC)
Jonathan Grossman, MD
Received Funding for Study: (Yes)
Conflicts of interest and financial disclosures relevant to the manuscript topic:
Institutional funding received through (Malova, Inc.)
Joseph Savino, MD
Received Funding for Study: (Yes)
Conflicts of interest and financial disclosures relevant to the manuscript topic:
Institutional funding received through (Vital Health Research, LLC)
References
- Manchikanti L, Kaye AM, Knezevic NN, Giordano J, Applewhite MK, Bautista A, et al. Comprehensive, evidence-based, consensus guidelines for prescription of opioids for chronic non-cancer pain from the American Society of Interventional Pain Physicians (ASIPP). Pain Physician. 2023 Dec;26(7 Suppl):S7-126. PMID: 38117465.
- Sembrano JN, Polly DW Jr. How often is low back pain not coming from the back? Spine (Phila Pa 1976). 2009 Jan 1;34(1):E27-32. doi:10.1097/BRS.0b013e31818b8882. PMID: 19127145.
- Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995 Jan 1;20(1):31-7. doi:10.1097/00007632-199501000-00007. PMID: 7709277.
- Falowski S, Sayed D, Pope J, Patterson D, Fishman M, Gupta M, et al. A review and algorithm in the diagnosis and treatment of sacroiliac joint pain. J Pain Res. 2020 Dec 8;13:3337-48. doi:10.2147/JPR.S279390. PMID: 33335420; PMCID: PMC7737553.
- Sayed D, Khatri N, Rupp A, Bovinet C, Azeem N, Li S, et al. Salvage of failed lateral sacroiliac joint fusion with a novel posterior sacroiliac fusion device: diagnostic approach, surgical technique, and multicenter case series. J Pain Res. 2022 May 12;15:1411-20. doi:10.2147/JPR.S357076. PMID: 35592816; PMCID: PMC9112175.
- Lorio MP, Polly DW Jr, Ninkovic I, Ledonio CG, Hallas K, Andersson G. Utilization of minimally invasive surgical approach for sacroiliac joint fusion in surgeon population of ISASS and SMISS membership. Open Orthop J. 2014 Jan 24;8:1-6. doi:10.2174/1874325001408010001. PMID: 24551025; PMCID: PMC3924210.
- Lee DW, Patterson DG, Sayed D. Review of current evidence for minimally invasive posterior sacroiliac joint fusion. Int J Spine Surg. 2021 Jun;15(3):514-24. doi:10.14444/8073. Epub 2021 May 7. PMID: 33963035; PMCID: PMC8176825.
- Kaye AD, Edinoff AN, Scoon L, Youn S, Farrell KJ, Kaye AJ, et al. Novel interventional techniques for chronic pain with minimally invasive arthrodesis of the sacroiliac joint: (INSITE, iFuse, Tricor, Rialto, and others). Rheumatol Ther. 2021 Sep;8(3):1061-72. doi:10.1007/s40744-021-00350-8. Epub 2021 Jul 30. PMID: 34331270; PMCID: PMC8380604.
- Martin CT, Haase L, Lender PA, Polly DW. Minimally invasive sacroiliac joint fusion: the current evidence. Int J Spine Surg. 2020 Feb 10;14(Suppl 1):20-9. doi:10.14444/6072. PMID: 32123654; PMCID: PMC7041666.
- Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013 May 17;7:163-8. doi:10.2174/1874325001307010163. PMID: 23730380; PMCID: PMC3664440.
- Sachs D, Capobianco R. One-year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012 Dec 27;6(1):13. doi:10.1186/1750-1164-6-13. PMID: 23270468; PMCID: PMC3561253.
- Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495-502. doi:10.2174/1874325001206010495. Epub 2012 Nov 30. PMID: 23284593; PMCID: PMC3529399.
- Smith AG, Capobianco R, Cher D, Rudolf L, Sachs D, Gundanna M, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013 Oct 30;7(1):14. doi:10.1186/1750-1164-7-14. PMID: 24172188; PMCID: PMC3817574.
- Shamrock AG, Patel A, Alam M, Shamrock KH, Al Maaieh M. The safety profile of percutaneous minimally invasive sacroiliac joint fusion. Global Spine J. 2019 Dec;9(8):874-80. doi:10.1177/2192568218816981. Epub 2019 Feb 14. PMID: 31819854; PMCID: PMC6882089.
- Polly DW, Swofford J, Whang PG, Frank CJ, Glaser JA, Limoni RP, et al; INSITE Study Group. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016 Aug 23;10:28. doi:10.14444/3028. PMID: 27652199; PMCID: PMC5027818.
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al; European Palliative Care Research Collaborative (EPCRC). Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011 Jun;41(6):1073-93. doi:10.1016/j.jpainsymman.2010.08.016. PMID: 21621130.
- Wewege MA, Jones MD, Williams SA, Kamper SJ, McAuley JH. Rescaling pain intensity measures for meta-analyses of analgesic medicines for low back pain appears justified: an empirical examination from randomised trials. BMC Med Res Methodol. 2022 Nov 4;22(1):285. doi:10.1186/s12874-022-01763-x. PMID: 36333665; PMCID: PMC9636623.
- Kucharzyk D, Colle K, Boone C, Araghi A. Clinical outcomes following minimally invasive sacroiliac joint fusion with decortication: the EVoluSIon clinical study. Int J Spine Surg. 2022 Feb;16(1):168-75. doi:10.14444/8185. Epub 2022 Feb 25. PMID: 35217586; PMCID: PMC9519080.
- Kube RA, Muir JM. Sacroiliac joint fusion: one-year clinical and radiographic results following minimally invasive sacroiliac joint fusion surgery. Open Orthop J. 2016 Nov 30;10:679-89. doi:10.2174/1874325001610010679. PMID: 28144378; PMCID: PMC5220174.
- Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008 Sep;90(9):1839-47. doi:10.2106/JBJS.G.01095. PMID: 18762642.
- Bae J, Lee SM, Lee SH, Shin SH, Kim HJ, Kim KH. The likelihood of reaching substantial clinical benefit after an interlaminar dynamic spacer for chronic low back pain: a clinical and radiologic analysis of a prospective cohort. World Neurosurg. 2017 May;101:589-98. doi:10.1016/j.wneu.2017.02.083. Epub 2017 Feb 27. PMID: 28242487.
- Endres S, Ludwig E. Outcome of distraction interference arthrodesis of the sacroiliac joint for sacroiliac arthritis. Indian J Orthop. 2013 Sep;47(5):437-42. doi:10.4103/0019-5413.118197. PMID: 24133301; PMCID: PMC3796914.
- Whang PG, Patel V, Duhon B, Sturesson B, Cher D, Carlton Reckling W, et al. Minimally invasive SI joint fusion procedures for chronic SI joint pain: systematic review and meta-analysis of safety and efficacy. Int J Spine Surg. 2023 Dec 26;17(6):794-808. doi:10.14444/8543. PMID: 37798076; PMCID: PMC10753354.
- Maxwell G, Lyon KA, Bhenderu LS, Schuchart G, Desai R. Sacral dysmorphism increases the risk of superior gluteal artery injury in percutaneous sacroiliac joint fusion: case report and literature review. Cureus. 2021 Nov 13;13(11):e19532. doi:10.7759/cureus.19532. PMID: 34934552; PMCID: PMC8668144.
- Laynes RA, Aravindan S, Wharton B, Layne JE, Kleck CJ, Patel VV. Injury to the superior gluteal artery during minimally invasive sacroiliac joint fusion: a case series. JBJS Case Connect. 2024 Sep 13;14(3). doi:10.2106/JBJS.CC.24.00202. PMID: 39270041.
- Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021 Feb 15;31(1):010502. doi:10.11613/BM.2021.010502. Epub 2020 Dec 15. PMID: 33380887; PMCID: PMC7745163.