A Systematic Review and Meta-Analysis on Anticoagulants and Vascular Calcification
Tejaswini Yeshala*,1, Anudeep Reddy Annem2, Pragna Gajawada3
1MBBS- Maheshwara Medical College
2MBBS-Kurnool Medical College
3Kazakh National Medical University, Almaty, Kazakhstan
*Corresponding Author: Tejaswini Yeshala, MBBS, Maheshwara Medical College, India
Received: 20 July 2025; Accepted: 31 July 2025; Published: 07 August 2025
Article Information
Citation: Tejaswini Yeshala, Anudeep Reddy Annem, Pragna Gajawada. A Systematic Review and Meta-Analysis on Anticoagulants and Vascular Calcification. Archives of Internal Medicine Research. 8 (2025): 233-245.
Share at FacebookAbstract
Countless patients who are being treated with vitamin K adversaries (VKA) with the end goal of anticoagulation additionally have vascular or valvular calcification simultaneously. As per the possibility that vascular and valvular calcification is a result of VKA treatment, the motivation behind this meta-investigation was to look at the hypothesis. We completed an extensive pursuit of the distributed writing to find concentrates on that gave proof of blood vessel or valvular calcification in people who were treated with VKA. This study utilized irregular impacts reverse difference models to explore the connections between's the use of VKA and calcification. The aftereffects of this examination were given as chances proportions (OR) and 95% certainty stretches (95% CI). Additionally, univariate meta-relapse examinations were completed to find any impact mediators that might have been available. Remembered for the examination were 35 investigations, totalling 15594. There was a middle subsequent time of 2.3 years, with an interquartile range (IQR) of 1.2-4.0 years. The mean age of the members was 66.2 ± 3.6 years, with a standard deviation of 3.6 years. Most of the members were male, with 77% (IQR: 72-95%). The utilization of VKA was connected with a raised chances proportion for coronary vein calcification [1.21 (1.08, 1.36), p = 0.001], which was alleviated by the length of treatment [meta-relapse coefficient B of 0.08 (0.03, 0.13), p = 0.0005]. Also, the presence of extra-coronary calcification, which affected the aorta, carotid supply route, bosom conduit, and veins of the lower limits, was seen to be raised in patients who were treated with VKA [1.86 (1.43, 2.42), p < 0.00001]. This increment was directed by the measurable changes of the impact assesses that were accounted for by the creator [B: - 0.63 (- 1.19), - 0.08], p = 0.016]. Regardless of the way that the effect of VKA on the calcification of the aortic valve was demonstrated to be significant [3.07 (1.90, 4.96), p < 0.00001], it is critical to take note of that these preliminaries were tormented by an enormous gamble of distribution inclination. The conceivable unfriendly impacts of VKA incorporate calcification of the vascular and valvular designs. To additionally research the clinical pertinence of these unfriendly effects on cardiovascular results, further speculation is required.
Keywords
Cardiovascular Calcifications, Atherosclerosis, Coronary Artery Disease, Breast Arterial Calcifications (BAC), Peripheral Arterial Disease (PAD), Aortic Calcification Index
Cardiovascular Calcifications articles, Atherosclerosis articles, Coronary Artery Disease articles, Breast Arterial Calcifications (BAC) articles, Peripheral Arterial Disease (PAD) articles, Aortic Calcification Index articles.
Article Details
Introduction
The way that vascular calcification is a free indicator of cardiovascular sickness (CVD) and mortality (1) is a finding that has gotten inescapable acknowledgment. Various investigations have shown a relationship among's calcification and clinically huge coronary course illness (computer aided design) (2-4), intense heart and cerebrovascular occasions (5, 6), blood vessel solidness and hypertension (7), and aortic valve sickness (8). Cardiovascular infection is the significant reason for death, representing in excess of about a third of all passings worldwide. With the coming of coronary corridor calcium scoring, a harmless imaging stage has developed with the end goal of atherosclerotic cardiovascular illness risk evaluation and the direction of lipid-bringing down drugs for essential counteraction (9, 10). During the 1950s, the anticoagulant known as warfarin, which is a vitamin K bad guy (VKA), was first brought into clinical practice (11). Warfarin and other VKAs have been authorized for the anticipation of thrombotic occasions in patients with repetitive venous apoplexy, atrial fibrillation, valvular coronary illness, and valve substitution (12). This endorsement has been conceded throughout the span of numerous years. Notwithstanding the way that the use of VKA has diminished throughout recent years because of the advancement of more secure non-vitamin K oral anticoagulants (NOAC or DOAC, which represents direct oral anticoagulants), VKAs keep on being widely given and are the sole drug that is shown by rules for patients who have prosthetic valves (13-16). Furthermore, patients who are more seasoned and patients who have been determined to have a few circumstances are bound to be endorsed warfarin for anticoagulation (17).
Through the concealment of vitamin K-subordinate post-translational gamma-carboxylation, which is essential for the movement of coagulation factors II, VII, IX, and X, as well as various different proteins, VKA can obstruct these proteins (18). Under typical physiological settings, calcification is restrained by various endogenous inhibitors. These inhibitors incorporate network Gla protein (MGP), pyrophosphate, and plasma fetuin-A (19). The gamma-carboxylation process is essential for the inhibitory capability of MGP, which is an individual from a similar gathering of gamma-carboxylated proteins as coagulation factors (18). It is guessed that the decline in vitamin K-subordinate gamma-carboxylation of MGP that happens because of drawn out use of VKA is answerable for the expanded vascular calcification that is related with its utilization (20, 21). There is as of now an absence of acknowledgment about the capability that VKA plays in vascular calcification, and the clinical significance of this not entirely settled (22). Introduced here is the absolute first meta-examination of clinical exploration that have been directed regarding this matter. In this review, our motivation was to offer information that upholds the speculation that the utilization of VKA is related with cardiovascular calcification.
Methods
Search strategy
There was no limitation put on the incorporation of a specific sign for the utilization of VKA; rather, all clinical examinations, except for contextual investigations and case series, were thought about. The essential results were calcification of the coronary courses, calcification of the corridors beyond the coronary conduits (like the stomach or thoracic aorta, carotid veins, bosom supply routes, and corridors of the limits), and calcification of the valve device. Between the dates of Walk 29, 2022 and Walk 29, 2022, the center efficient writing search was completed in PubMed utilizing a stepwise catchphrase search technique (Beneficial Table 1). A bunch of PubMed channels was applied to the query items to reject surveys, case reports, proposals, and exploration conventions from the gathering. Also thought about were the reference arrangements of the pertinent papers as well as the "comparative articles" that were suggested by PubMed. Different data sets, for example, CINAHL, the Cochrane Register of Studies, and Google Researcher, were looked through to track down additional references. The accompanying incorporation standards were applied to the digests: (1) treatment with VKA; and (2) the presence of no less than one blood vessel or valvular calcification result, for example, calcium score or record, calcified plaque volume, presence or nonappearance of calcification, calcification seriousness grade, or a yearly pace of movement. The digests were audited by two specialists, and any distinctions that emerged were dealt with by getting everybody to settle on something.
Data extraction and management
The determination of the information depended on an examination of the general text. The information that was extricated incorporated a review identifier, the country, the review plan, the example size, the mean or middle age, the level of guys, the term of VKA treatment, the calcification outcome(s), the strategies for evaluation of calcification, the impact size gauges, and a concise portrayal of the measurable models that were utilized for the impact gauges. Occurrence, pervasiveness, chances proportions (OR), mean change from gauge, relapse coefficients, proportions of anticipated counts (REC), and F insights were the factual measures that were utilized to separate the meaning of the effect sizes. coronary course calcium (CAC) score, which was surveyed by registered tomography (CT), calcified plaque volume, still up in the air by coronary CT angiography (CCTA), and coronary supply route calcium file, which was gained by intravascular ultrasonography (IVUS) were the consequences of the coronary review. The presence or nonattendance of calcification, seriousness grade, calcification score, or a yearly pace of (not entirely set in stone by CT, X-beam, mammography, or histology) were all instances of extra-coronary results. All in all, the outcomes of calcification of the aortic valve contained the presence or nonattendance of calcification on transthoracic ultrasonography (US), the quantity of aortic valve flyers that were harmed, the CT calcification score, or positive outcomes on histology.
Statistical Analysis
The Survey Chief PC device, rendition 5.4 (RevMan5, the Cochrane Joint effort, 2020), was utilized to do the investigation on the information base. Utilizing either the RevMan5 impact size adding machine or an internet based impact size mini-computer apparatus [Practical Meta-Examination Impact Size Number cruncher (25)], the impact sizes were addressed as chances proportions (OR) and standard blunders (SE). Information amalgamation was achieved through the utilization of backwards change irregular impacts models. To order the investigations, coronary, extra-coronary, and valvular calcification were the three classifications that were utilized. The joined evaluations were figured as chances proportions (OR) and certainty stretches (95% CI) comparative with the presence of vascular or valvular calcification in patients who were treated with VKA in contrast with patients who were not treated with VKA. This gathering of patients included patients who were treated with anticoagulants that were not related with VKA as well as patients who had no signs for anticoagulation and were not given any anticoagulants. Using the I2 test that was registered in RevMan5, the factual heterogeneity was examined and characterized. Utilizing the Meta-Fundamentals program, which was gotten on February 9, 2022 from https://www.erim.eur.nl/research-support/meta-basics, an Egger relapse and Begg and Mazumdar rank relationship test were acted to assess the potential for distribution inclination (26). Also, the Meta-Basics instrument was used for the univariate meta-relapse investigations. These examinations considered the time of distribution, the geological district (mainland), the review plan, the example size, the patient attributes, the middle age, the proportion of members by sex, the length of the VKA treatment, the calcium imaging methodology, and whether the impact gauges were adapted to frustrating elements. Each critical modifier that was found by meta-relapse was utilized to direct the sub-bunch concentrates on that were completed. To additional intricate, the responsiveness examination was completed by disposing of each exploration in turn from the meta-examination that compared to it. An importance level of p < 0.05 was taken on for the review.
Results
Search results and characteristics of the included studies
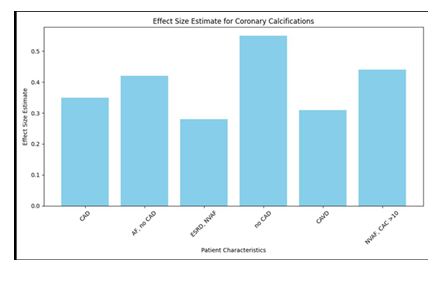
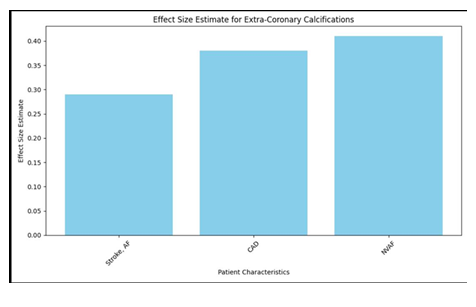
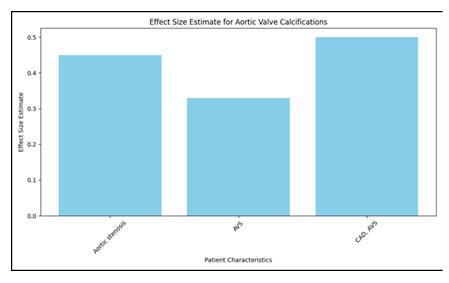
In the wake of doing a pursuit on PubMed, a sum of 330 papers were found, and five additional articles were gotten from their separate sources. These 335 pieces included 114 audits and publications, 22 case reports, two review conventions, and two clinical proposals. Moreover, there were two clinical rules. Another 152 were proclaimed immaterial by an understanding arrived at between two specialists (NDK and OVS) on the off chance that the papers didn't have a place with human subjects, didn't give VKA treatment or impact gauges, or didn't have a normalized strategy of distinguishing and estimating calcification. Following an assessment of the total texts of the excess 43 distributions, seven extra were ignored for the accompanying reasons: (1) they needed information (n = 1), they didn't meet the incorporation measures (n = 4), they were an optional examination of a generally included research (n = 2), or they were a continuous examination (n = 1). This brought about the consideration of 35 preliminaries and 15,594 individuals in the examination, of whom 6,251 were treated with VKA (27-61). There were a sum of 32 observational examinations, with 38 partners, 15,261 members, and 6,082 VKA clients. Three of these investigations were randomized preliminaries, with four unmistakable examination associates, 333 patients, and 169 VKA clients (29, 32, 39). The other 32 investigations were observational examinations (27, 28, 30, 31, 33-38, 40-61). Thirteen exploration were directed to investigate the impacts of VKA on coronary corridor calcification. These examinations went from 27 to 38 and included 15 accomplices, 23,768 members, and 2,625 people who got VKA. 18 accomplices, 4,740 members, and 1,595 patients who were treated with VKA (40-54) were remembered for the sixteen examinations that showed extra-coronary calcification, which alludes to calcification in any course other than the coronary supply route. Finally, nine examinations (31, 49, 55-61) were led to survey the impacts of VKA drug on aortic valve calcification. The all out number of members in these examinations was 15,594. Table 1 shows the attributes of the examinations that were remembered for the investigation.
From 2005 to 2022, studies were distributed in scholastic diaries. In Europe, twenty examinations were completed (29-31, 34-36, 38, 41-43, 46-51, 53, 55, 57-59), thirteen investigations were done in North America (27, 28, 32, 33, 37, 39, 40, 44, 45, 52, 56, 58, 60), and two examinations were done in Asia (54, 61). As referenced before, we tracked down three randomized preliminaries (29, 32, 39), one meta-investigation of patient-level information from eight randomized preliminaries (27), 21 review accomplice studies (30, 31, 34-38, 40-42, 45, 48-52, 54, 55, 57, 59, 60), one forthcoming partner (61), and nine cross-sectional examinations (28, 33, 43, 44, 46, 47, 53, 56, 58). These examinations were led in the US. The quantity of members in the example fluctuated from 37 to 17,254 altogether. The interquartile range (IQR) of the example size went from 108 to 387 patients, with 207 being the middle example size. 77% (interquartile range: 72-95%) of the members were men, and the typical age of the members was 66.2 years with a standard deviation of 3.6 years. There were 33 gatherings of patients who were either tentatively or reflectively followed, and the weighted middle length of VKA prescription was 2.3 years (interquartile range somewhere in the range of 1.2 and 4.0) (27, 29-32, 34-42, 45, 48-52, 54, 55, 57, 59-61). Cross-sectional examinations were led on nine different exploration partners, and the length of treatment was not shown for any of them (28-58).
Quality assessment
There were three randomized preliminaries, and one of them was a "per-convention" study (29), which had a critical gamble of predisposition inferable from missing result information. Then again, two extra "aim to-treat" studies had issues about missing result information (32) and specific detailing (32, 39) (Advantageous Table 2). An assessment of the potential for predisposition in observational examinations was done utilizing a Newcastle-Ottawa quality scale with nine places. Notwithstanding, there were five examinations in which the gamble of predisposition was appraised low to direct on the Newcastle-Ottawa quality scale (31, 33, 44, 47, 53) (Beneficial Table 3). Most of the examinations were of basically moderate quality (27, 28, 30, 31, 34-38, 40-43, 45, 46, 48-59, 61) [median score 7 (interquartile range 5-9)]. The impacts of the utilization of VKA on the calcification of the coronary, extra-coronary, and aortic valves. There was a relationship between's the use of VKA and an expansion in vascular and valvular calcification. Contrasting patients who were not treated and VKA to the individuals who were treated with VKA, the chances proportion (OR) for coronary corridor calcification was 1.21 (95% certainty stretch [CI]: 1.08, 1.36), with a p-worth of 0.001. Moreover, the utilization of VKA was connected to the presence of extra-coronary vascular calcification in the corridors of the aorta, carotid conduits, bosom veins, and courses of the lower limits [odds proportion 1.86 (1.43, 2.42), p < 0.00001]. Moreover, we found a relationship between's the utilization of VKA and the improvement of aortic valve calcification, [odds proportion 3.07 (1.90, 4.96), p < 0.00001]. The discoveries of the coronary (n = 15), extra-coronary vascular (n = 18), and aortic valve (n = 9) examinations uncovered that the degree of heterogeneity between the investigations was genuinely critical at I2 upsides of 69, 78, and 90%, separately.
Table 1: Characteristics of the included studies
Meta-regression and subgroup analysis
The distinguishing proof of conceivable effect modifiers was achieved through the utilization of meta-relapse investigation. We utilized univariate arbitrary impacts relapses to dissect the impacts of the accompanying variables: the extended time of distribution, the geographic district (landmass), the review plan, the example size, the patient attributes, the middle age, the proportion of members by sex, the span of VKA treatment, the calcium imaging methodology, and whether the impact gauges were accounted for adapted to the bewildering factors (Table 1). The appraisals of coronary supply route calcification were impacted by three logical factors. The extended time of distribution had a B relapse coefficient of - 0.04 (95% certainty stretch: - 0.08, 0.00), with a p-worth of 0.035. Moreover, the orientation proportion communicated as a level of male members had a B worth of - 0.01 (- 0.03, 0.00), with a p-worth of 0.039. Then again, the length of VKA treatment had a B worth of 0.08 (0.03, 0.13), with a p-worth of 0.0005 to be viewed as in Table 2. It was seen that the consequences for the extra-coronary vascular calcification were changed relying upon whether the gave gauges were amended to the bewildering factors [B = - 0.63 (- 1.19), - 0.08; p = 0.016, Table 2]. We led a meta-relapse examination and found that the impact gauges were conceivably impacted by the example size [B = - 0.32 (- 2.35, - 0.04), p = 0.009, Table 2]. This was in spite of the way that the quantity of examinations relating to aortic valves was moderately low (n = 9), and there was an impressive gamble of distribution predisposition. Thus, we completed subgroup examinations in which we thought about the top portion of the investigations to the base portion of the investigations concerning every one of the recognized modifiers (distribution year, sex proportion, length of VKA treatment, and factual change). We found that the effect of the length of VKA on the calcification of the coronary conduits was very nearly being critical while contrasting investigations and a more drawn out term (>1.7 years) to review with a span of ≤1.7 years (bunches distinction test I2 = 72%, p = 0.06; Table 3). We additionally found that, as was normal by meta-relapse, the appraisals of VKA impacts of the extra-coronary calcification were modified relying upon whether the introduced models were adapted to likely bewildering factors.
Discussion
The motivation behind this precise survey, which has an emphasis on randomized controlled preliminaries and contains a significant example size of 15,594 individuals, was to explore the connection among anticoagulants and blood vessel (and valvular) calcification. Our discoveries show that there is a significant relationship between the utilization of vitamin K bad guys (VKAs) and expanded blood vessel and valvular calcification. This is rather than the way that different anticoagulants didn't exhibit a relationship of a similar kind.
Table 2: Coronary Calcifications
Table 3: Extra-Coronary Calcifications
Table 4: Aortic Valve Calcifications
To stay away from calcification in the veins and heart valves, vitamin K-subordinate proteins are totally fundamental. Vitamin K bad guys, like warfarin, block the actuation of these proteins. As per the system of activity that VKAs are accepted to have, it is accepted that they accelerate the course of calcification. This thought is affirmed by past examination, which our review approves. More specifically, VKAs block the network Gla-protein (MGP), which is a strong inhibitor of vascular calcification, from being carboxylated. Calcification of the vascular and valvular designs happens all the more rapidly when MGP isn't completely carboxylated, which prompts a lessening in the inhibitory impact of the compound. There have been countless observational examinations assessing the impacts of VKA on vascular calcification that have been accounted for. These investigations are of a top notch. In these preliminaries, calcification was assessed in many people who had been treated with VKA. The utilization of calcium imaging in demonstrative examinations, along with the advancement of further developed imaging modalities, made it conceivable to lead more exact examinations on greater patient associates. The end of the conceivable bewildering factors was achieved by various journalists by means of the utilization of a penchant matching methodology. Still others involved extra broad factual methodologies to limit the effect of bewildering factors on calcification assessments. Furthermore, the nature of distributions on the use of VKA and vascular calcification is upheld by the shortfall of a significant gamble of distribution inclination and the way that we had the option to distinguish the length of treatment as a modifier of the effect gauges. To wrap things up, the assessment of calcification in the primary no holds barred randomized preliminaries with VKA was made conceivable by the new send off of another class of non-vitamin K oral anticoagulants known as NOAC. This was the situation in spite of the way that every one of the three randomized examinations assessed a similar peculiarity.
As indicated by our exploration, in any case, there was no perceptible connection between vascular or valvular calcification and non-vitamin K oral anticoagulants (NOACs), which incorporate direct thrombin inhibitors and variable Xa inhibitors. This was the situation. There are different instruments that are answerable for the working of these anticoagulants. These exercises don't obstruct the digestion of vitamin K or the carboxylation of macrocyclic G-protein. The meaning of this differential lies in the way that it exhibits how NOACs might be more useful than VKAs for people who are in danger of encountering complexities connected with calcification issues. This information is useful for going with choices applicable to clinical practice. It is vital to take note of that the aftereffects of the survey have significant clinical outcomes. With regards to the cardiovascular results of patients, the decision of an anticoagulant for long haul use might have a critical effect, especially on patients who are at a high gamble of blood vessel or valvular calcification. Clinical experts should give cautious thought to both the dangers and advantages related with VKAs, especially in populaces where calcification is an issue. VKAs have been related with an expansion in calcification, which features the need of cautious checking and, in certain cases, the investigation of elective anticoagulant treatments in patients who are vulnerable. Moreover, it raises the issue of whether patients who need VKAs yet can't change to NOACs would profit from supplemental treatment that is pointed toward decreasing how much calcification that happens in their bodies. While it is vital to recognize the constraints of this audit, which remember changeability for research plans, socioeconomics, and calcification appraisal techniques, it is additionally essential to recognize that this assessment gives educational data. There will be an improvement in the likeness of results in future examination assuming that these boundaries are normalized. Furthermore, research that is led over a more extended timeframe is fundamental to see the value in the effect of anticoagulant medicine on the general movement of calcification and clinical results completely. Furthermore, it is the very pinnacle of need to accomplish other things research to investigate the basic systems that are liable for the calcification that is brought about by VKAs and to make practical medicines that could diminish the seriousness of this effect. Almost certainly, new treatment choices might open up assuming researchers examine the job that vitamin K admission from food plays and the possible advantages of vitamin K supplementation in individuals with VKA.
Conclusion
Because of the reasonable association that exists between these medications and expanded blood vessel and valvular calcification, our far reaching investigation has reached the resolution that patients who need long haul anticoagulation with vitamin K bad guys (VKAs) face a huge gamble. Then again, it doesn't appear to be that different anticoagulants have a similar unfriendly impact as the one recently referenced. Future examination tries that are pointed toward working on understanding results according to anticoagulant medication ought to be directed by the discoveries of this review, which ought to help direct such undertakings.
References
- Shekar C, Budoff M. Calcification of the heart: mechanisms and therapeutic avenues. Exp Rev Cardiovasc Therapy. (2018) 16:527–36. doi: 10.1080/14779072.2018.1484282
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-T
- Breen JF, Sheedy PF 2nd, Schwartz RS, Stanson AW, Kaufmann RB, Moll PP, et al. Coronary artery calcification detected with ultrafast CT as an indication of coronary artery disease. Radiology. (1992) 185:435–9. doi: 10.1148/radiology.185.2.1410350
- Margolis JR, Chen JT, Kong Y, Peter RH, Behar VS, Kisslo JA. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. (1980) 137:609–16. doi: 10.1148/radiology.137.3.7444045
- Sugiyama T, Yamamoto E, Fracassi F, Lee H, Yonetsu T, Kakuta T, et al. Calcified plaques in patients with acute coronary syndromes. JACC Cardiovasc Interv. (2019) 12:531–40. doi: 10.1016/j.jcin.2018.12.013
- Iwai S, Watanabe M, Okamura A, Kyodo A, Nogi K, Kamon D, et al. Prognostic impact of calcified plaque morphology after drug eluting stent implantation- an optical coherence tomography study. Circ J. (2021). 85:2019–28. doi: 10.1253/circj.CJ- 20-1233
- Aladin AI, Al Rifai M, Rasool SH, Dardari Z, Yeboah J, Nasir K, et al. Relation of coronary artery calcium and extra -coronary aortic calcium to incident hypertension (from the multi-ethnic study of atherosclerosis). Am J Cardiol. (2018) 121:210–6. doi:10. 1016/j.amjcard.2017.10.018
- Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. (2015) 66:561 –77. doi:10. 1016/j.jacc.2015.05.066
- Golub I, Lakshmanan S, Dahal S, Budoff MJ. Utilizing coronary artery calcium to guide statin use. Atherosclerosis. (2021) 326:17–24. doi: 10.1016/j.atherosclerosis.2021.04.011
- Grundy SM, Stone NJ, Bailey A AL, Beam C, Bi Birtcher KK Blumenthal RS, et al. 2018
- guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139:e1082–43. doi: 10.1161/CIR.00000000000 00624
- Nicholson JH. Clinical experiences with anticoagulants; a comparison of coumadin (warfarin) sodium and dicumarol (bishydroxycoumarin). Angiology. (1957) 8:456–65. doi: 10.1177/000331975700800508
- Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. (1999) 59:635–46.
- Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Advances. (2020) 4:4693–738. doi: 10.1182/bloodadvances.2020001830
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
- Wetmore JB, Roetker NS, Yan H, Reyes JL, Herzog CA. Direct- acting oral anticoagulants versus warfarin in medicare patients with chronic kidney disease and atrial fibrillation. Stroke. (2020) 51:2364–73. doi: 10.1161/STROKEAHA.120.028934
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
- Mills C, Snider MJ, Ortman TC, Dush A, Hevezi MS, Li J, et al. Trends in anticoagulation management services following incorporation of direct oral anticoagulants at a large academic medical center. J Thromb Thrombol. (2021) 51:1050–8. doi:10. 1007/s11239-020-02286-2
- Willems BA, Vermeer C, Reutelingsperger CP, Schurgers LJ. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. (2014) 58:1620–35. doi: 10.1002/mnfr.201300743
- Bäck M, Aranyi T, Cancela ML, Carracedo M, Conceição N, Leftheriotis G, et al. Endogenous calcification inhibitors in the prevention of vascular calcification: a consensus statement from the COST action EuroSoftCalcNet. Front Cardiovasc Med. (2018) 5:196. doi: 10.3389/fcvm.2018.00196
- Kaesler N, Schurgers LJ, Floege J. Vitamin K and cardiovascular complications in chronic kidney disease patients. Kidney Int. (2021). 100:1023–36. doi: 10.1016/j.kint.2021.06.037
- Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, et al. Novel conformation-specific antibodies against matrix gamma- carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscl Thromb Vasc Biol. (2005) 25:1629–33. doi: 10.1161/01.ATV.0000173313.46222.43
- van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of vitamin K antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients. (2015) 7:9538–57. doi: 10.3390/nu7115479
- Sterne JAC, Savovic´ J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
- Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2013). Available online at: https://www.ohri.ca//programs/ clinical_epidemiology/oxford.asp (accessed March 29, 2022).
- Wilson D. Practical Meta-Analysis Effect Size Calculator [Online calculator] Available online at: https://campbellcollaboration.org/research-resources/effect- size-calculator.html (accessed March 29, 2022).
- Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta- analysis. Res Synthesis Meth. (2017) 8:537–53. doi: 10.1002/jrsm.1260
- Andrews J, Psaltis PJ, Bayturan O, Shao M, Stegman B, Elshazly M, et al. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imag. (2018) 11:1315–23. doi:10. 1016/j.jcmg.2017.04.010
- Chaikriangkrai K, Valderrabano M, Bala SK, Alchalabi S, Graviss EA, Nabi F, et al. Prevalence and implications of subclinical coronary artery disease in patients with atrial fibrillation. Am J Cardiol. (2015) 116:1219–23. doi: 10.1016/j.amjcard.2015.07.041
- De Vriese AS, Caluwe R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin k antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the valkyrie study. J Am Soc Nephrol. (2020) 31:186–96. doi: 10.1681/ASN.2019060579
- [31]. Hasific S, Øvrehus KA, Gerke O, Hallas J, Busk M, Lambrechtsen J, et al. Extent of arterial calcification by conventional vitamin K antagonist treatment. PLoS ONE. (2020) 15:e0241450. doi: 10.1371/journal.pone.0241450
- Koos R, Mahnken AH, Mühlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. (2005) 96:747–9. doi:10. 1016/j.amjcard.2005.05.014
- Lee J, Nakanishi R, Li D, Shaikh K, Shekar C, Osawa K, et al. Randomized trial of rivaroxaban versus warfarin in the evaluation of progression of coronary atherosclerosis. Am Heart J. (2018) 206:127–30. doi: 10.1016/j.ahj.2018. 08.007
- Palaniswamy C, Aronow WS, Sekhri A, Adapa S, Ahn C, Singh T, et al. Warfarin use and prevalence of coronary artery calcification assessed by multislice computed tomography. Am J Ther. (2014) 21:148–51. doi: 10.1097/MJT.0b013e318249a1c6
- Plank F, Beyer C, Friedrich G, Stühlinger M, Hintringer F, Dichtl W, et al. Influence of vitamin K antagonists and direct oral anticoagulation on coronary artery disease: a CTA analysis. Int J Cardiol. (2018) 260:11–5. doi: 10.1016/j.ijcard.2018.03.019
- Schurgers LJ, Joosen IA, Laufer EM, Chatrou ML, Herfs M, Winkens MH, et al. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS ONE. (2012) 7:e43229. doi: 10.1371/journal.pone.0043229
- Ünlü S, Sahinarslan A, Kiliç HK, Gökalp G, Sezenöz B, Erba¸s G, et al. Long- term vitamin-K antagonist use and coronary artery calcification. Herz. (2020) 45:580–5. doi: 10.1007/s00059-018-4760-9
- Villines TC, O’Malley PG, Feuerstein IM, Thomas S, Taylor AJ. Does prolonged warfarin exposure potentiate coronary calcification in humans? Results of the warfarin and coronary calcification study. Calcif Tissue Int. (2009) 85:494–500. doi: 10.1007/s00223-009-9300-4
- Weijs B, Blaauw Y, Rennenberg RJ, Schurgers LJ, Timmermans CC, Pison L, et al. Patients using vitamin K antagonists show increased levels of coronary calcification: an observational study in low-risk atrial fibrillation patients. Eur Heart J. (2011) 32:2555–62. doi: 10.1093/eurheartj/ehr226
- Win TT, Nakanishi R, Osawa K, Li D, Susaria SS, Jayawardena E, et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am Heart J. (2019) 212:129–33. doi: 10.1016/j.ahj.2019.02.014
- Alappan HR, Kaur G, Manzoor S, Navarrete J, O’Neill WC. Warfarin accelerates medial arterial calcification in humans. Arterioscl Thromb Vasc Biol. (2020) 40:1413–9. doi: 10.1161/ATVBAHA.119.313879
- Eren Sadioglu R, Ustuner E, Ergun I, Ecder ST, Nergizoglu G, Keven K. Warfarin is associated with the risk of vascular calcification in abdominal aorta in hemodialysis patients: a multicenter case-control study. Turk J Med Sci. (2021). 51:2607–15. doi: 10.3906/sag-2104-221
- Fusaro M, Tripepi G, Noale M, Plebani M, Zaninotto M, Piccoli A, et al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol. (2015) 13:248–58. doi: 10.2174/15701611113119990146
- Fusaro M, Gallieni M, Rebora P, Rizzo MA, Luise MC, Riva H, et al. Atrial fibrillation and low vitamin D levels are associated with severe vascular calcifications in hemodialysis patients. J Nephrol. (2016) 29:419–26. doi: 10.1007/s40620-015-0236-7
- Han KH, Hennigar RA, O’Neill WC. The association of bone and osteoclasts with vascular calcification. Vasc Med. (2015) 20:527 –33. doi: 10.1177/1358863X15597076
- Han KH, O’Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc. (2016) 5:e0 02665. doi: 10.1161/JAHA.115.002665
- Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. (2009) 24:948–55. doi: 10.1093/ndt/gfn571
- Jean G, Chazot C, Bresson E, Zaoui E, Cavalier E. High serum sclerostin levels are associated with a better outcome in haemodialysis patients. Nephron. (2016) 132:181–90. doi: 10.1159/000443845
- Nuotio K, Koskinen SM, Mäkitie L, Tuimala J, Ijäs P, Heikkilä HM, et al. Warfarin treatment is associated to increased internal carotid artery calcification. Front Neurol. (2021) 12:696244. doi: 10.3389/fneur.2021.696244
- Peeters F, Dudink E, Kimenai DM, Weijs B, Altintas S, Heckman LIB, et al. Vitamin K antagonists, non-vitamin K antagonist oral anticoagulants, and vascular calcification in patients with atrial fibrillation. TH Open. (2018) 2:e391–e8. doi: 10.1055/s-0038-1675578
- Peeters MTJ, Houben R, Postma AA, van Oostenbrugge RJ, Schurgers LJ, Staals J. Vitamin K antagonist use and risk for intracranial carotid artery calcification in patients with intracerebral hemorrhage. Front Neurol. (2019) 10:1278. doi: 10.3389/fneur.2019.01278
- Rennenberg RJ, van Varik BJ, Schurgers LJ, Hamulyak K, Ten Cate H, Leiner T, et al. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. (2010) 115:5121–3. doi: 10.1182/blood-2010-01-264598
- Tantisattamo E, Han KH, O’Neill WC. Increased vascular calcification in patients receiving warfarin. Arterioscl Thromb Vasc Biol. (2015) 35:237–42. doi: 10.1161/ATVBAHA.114.304392
- Van Berkel B, Van Ongeval C, Van Craenenbroeck AH, Pottel H, De Vusser K, Evenepoel P. Prevalence, progression and implications of breast artery calcification in patients with chronic kidney disease. Clin Kidney J. (2022) 15:295 –302. doi:10. 1093/ckj/sfab178
- Wei N, Lu L, Zhang H, Gao M, Ghosh S, Liu Z, et al. Warfarin accelerates aortic calcification by upregulating senescence -associated secretory phenotype maker expression. Oxid Med Cell Longev. (2020) 2020:2043762. doi: 10.1155/2020/2043762
- Di Lullo L, Tripepi G, Ronco C, D’Arrigo G, Barbera V, Russo D, et al. Cardiac valve calcification and use of anticoagulants: preliminary observation of a potentially modifiable risk factor. Int J Cardiol. (2019) 278:243–9. doi: 10.1016/j.ijcard.2018.11.119
- Ing SW, Mohler Iii ER, Putt ME, Torigian D, Leonard MB. Correlates of valvular ossification in patients with aortic valve stenosis. Clin Transl Sci. (2009) 2:431–5. doi: 10.1111/j.1752-8062.2009.00168.x
- Koos R, Krueger T, Westenfeld R, Kühl HP, Brandenburg V, Mahnken AH, et al. Relation of circulating matrix gla-protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. (2009) 101:706–13. doi: 10.1160/TH08-09-0611
- Lerner RG, Aronow WS, Sekhri A, Palaniswamy C, Ahn C, Singh T, et al. Warfarin use and the risk of valvular calcification. J Thromb Haemost. (2009) 7:2023–7. doi: 10.1111/j.1538-7836.2009.03630.x
- Sonderskov PS, Lindholt JS, Hallas J, Gerke O, Hasific S, Lambrechtsen J, et al. Association of aortic valve calcification and vitamin K antagonist treatment. Eur Heart J Cardiovasc Imag. (2020) 21:718–24. doi: 10.1093/ehjci/jeaa065
- Tastet L, Pibarot P, Shen M, Clisson M, Côté N, Salaun E, et al. Oral anticoagulation therapy and progression of calcific aortic valve stenosis. J Am Coll Cardiol. (2019) 73:1869–71. doi: 10.1016/j.jacc.2019.01.043
- Yamamoto K, Koretsune Y, Akasaka T, Kisanuki A, Ohte N, Takenaka T, et al. Effects of vitamin K antagonist on aortic valve degeneration in non-valvular atrial fibrillation patients: prospective 4-year observational study. Thromb Res. (2017) 160:69–75. doi: 10. 1016/j.thromres.2017.10.027
- Kauppila L, Polak J, Cupples L, Hannan M, Kiel D, Wilson P. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25- year follow-up study. Atherosclerosis. (1997) 132:245–50. doi: 10.1016/S0021-9150(97)00106-8
- Witteman JC, Grobbee DE, Valkenburg HA, van Hemert AM, Stijnen T, Burger H, et al. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet. (1994) 343:504–7. doi: 10.1016/S0140-6736(94)91459-1
- Levy RJ, Schoen FJ, Levy JT, Nelson AC, Howard SL, Oshry LJ. Biologic determinants of dystrophic calcification and osteocalcin deposition in glutaraldehyde-preserved porcine aortic valve leaflets implanted subcutaneously in rats. Am J Pathol. (1983) 113:143–55.
- Taybi H, Capitanio MA. Tracheobronchial calcification: an observation in three children after mitral valve replacement and warfarin sodium therapy. Radiology. (1990) 176:728–30. doi: 10.1148/radiology.176.3.2389031
- Yoshida S, Torikai K. The effects of warfarin on calcinosis in a patient with systemic sclerosis. J Rheumatol. (1993) 20:1233–5.
- Jurgens PT, Carr JJ, Terry JG, Rana JS, Jacobs DR, Duprez DA. Association of abdominal aorta calcium and coronary artery calcium with incident cardiovascular and coronary heart disease events in black and white middle‐ aged people: the coronary artery risk development in young adults study. J Am Heart Assoc. (2021) 10:e023037. doi: 10.1161/JAHA.121.023037
- Takayama Y, Yasuda Y, Suzuki S, Shibata Y, Tatami Y, Shibata K, et al. Relationship between abdominal aortic and coronary art ery calcification as detected by computed tomography in chronic kidney disease patients. Heart Vessels. (2016) 31:1030–7. doi: 10.1007/s00380-015-0712-y
- [70]. Minssen L, Dao TH, Quang AV, Martin L, Andureau E, Luciani A, et al. Breast arterial calcifications on mammography: a new marker of cardiovascular risk in asymptomatic middle age women? Eur Radiol. (2022) 32:4889–97. doi: 10.1007/s00330-022-08571-3
- Nasir K, Katz R, Al-Mallah M, Takasu J, Shavelle DM, Carr JJ, et al. Relationship of aortic valve calcification with coronary artery calcium severity: the multi-ethnic study of atherosclerosis (MESA). J Cardiovasc Comp Tomog. (2010) 4:41–6. doi: 10.1016/j.jcct.2009.12.002
- Tintut Y, Honda HM, Demer LL. Biomolecules orchestrating cardiovascular calcification. Biomolecules. (2021) 11:1482. doi: 10.3390/biom11101482
- Aikawa E, Blaser MC, Jeffrey M. Hoeg award lecture: calcifying extracellular vesicles as building blocks of microcalcifications in cardiovascular disorders. Arterioscl Thromb Vasc Biol. (2021) 41:117–27. doi: 10.1161/ATVBAHA.120.314704
- Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. (1976) 35:156–62.
- Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. (2013) 12:576–83. doi: 10.1038/nmat3627
- Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imag. (2013) 6:747–54. doi: 10.1161/CIRCIMAGING.113.000382
- Chowdhury MM, Tarkin JM, Albaghdadi MS, Evans NR, Le EPV, Berrett TB, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease: a prospective clinical study. JACC Cardiovasc Imag. (2020) 13:1008–17. doi: 10.1016/j.jcmg.2019.03.031
- Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. (2012) 125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052
- Bjørklund G, Svanberg E, Dadar M, Card DJ, Chirumbolo S, Harrington DJ, et al. The role of matrix gla protein (MGP) in vascular calcification. Curr Med Chem. (2020) 27:1647–60. doi: 10.2174/0929867325666180716 104159
- Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, et al. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc. (2019) 8:e012141. doi: 10.1161/JAHA.119.012141
- Lee GP, Kim HL. Incremental value of the measures of arterial stiffness in cardiovascular risk assessment. Rev Cardiovasc Med. (2022) 23:6. doi: 10.31083/j.rcm2301006
- Vasan RS, Pan S, Xanthakis V, Beiser A, Larson MG, Seshadri S, et al. Arterial stiffness and long-term risk of health outcomes: the framingham heart study. Hypertension. (2022) 79:1045–56. doi: 10.1161/HYPERTENSIONAHA.121.18776
- Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, et al. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the dallas heart study. J Cardiovasc Magn Reson. (2014) 16:33. doi: 10.1186/1532-429X-16-33
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscl Thromb Vasc Biol. (2000) 20:1262–75. doi: 10.1161/01.ATV.20.5.1262
- Høilund-Carlsen PF, Sturek M, Alavi A, Gerke O. Atherosclerosis imaging with (18)F-sodium fluoride PET: state-of-the-art review. Eur J Nucl Med Mol Imag. (2020) 47:1538–51. doi: 10.1007/s00259-019-04603-1
- Nishimura S, Izumi C, Nishiga M, Amano M, Imamura S, Onishi N, et al. Predictors of rapid progression and clinical outcome of asymptomatic severe aortic stenosis. Circ J. (2016) 80:1863–9. doi: 10.1253/circj.CJ-16-0333
- Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. (2000) 343:611–7. doi: 10.1056/NEJM200008313430903
- Poterucha TJ, Goldhaber SZ. Warfarin and vascular calcification. Am J Med. (2016) 129:635.e1–4. doi: 10.1016/j.amjmed.2015.11.032
© 2016-2025, Copyrights Fortune Journals. All Rights Reserved