Active Surveillance of Adverse Events with Particular Interest Following Vaccination Against COVID-19 in Burkina Faso
Zoungrana KA1*, Koala D1, Bountogo M2, Ouédraogo I3, Yougbaré R3, Ouédraogo TR3, Diao R3, Sana R3, Gourmanon C4, Agawa A5, Rychen M5, Ahawo K5, Essoh A1, Tall H1
1Agence de Médecine Préventive (AMP), Ouagadougou, Burkina Faso
2Centre de Recherche en Santé de Nouna (CRSN), Nouna, Burkina Faso
3Ministère de la Santé et de l’Hygiène Publique, Burkina Faso
4Independent Consultant, Burkina Faso
5Global Alliance for Vaccine Initiative (GAVI), Burkina Faso
*Corresponding author: Koudmanégré Augustin ZOUNGRANA, Agence de Médecine Préventive, Ouagadougou, Burkina Faso.
Received: 13 August 2025; Accepted: 22August 2025; Published: 03 September 2025
Article Information
Citation: Zoungrana KA, Koala D, Bountogo M, Ouédraogo I, Yougbaré R, Ouédraogo TR, Diao R, Sana R, Gourmanon C, Agawa A, Rychen M, Ahawo K, Essoh A1, Tall H. Active Surveillance of Adverse Events with Particular Interest Following Vaccination Against COVID-19 in Burkina Faso. Archives of Clinical and Biomedical Research. 9 (2025): 359-369.
Share at FacebookAbstract
Introduction: Coronavirus 2019 or COVID-19 disease caused by SARSCoV- 2 first emerged in Wuhan, China. In Africa, there were 8,580,381 confirmed cases at the end of March 2022. Burkina Faso had 20,858 confirmed cases and 382 deaths in April 2022. Like other countries, vaccination is used as the main means of disease control. Rumors of vaccination-related side effects limit its uptake. This study aims to evaluate the safety of COVID-19 vaccines among vaccinated people in Burkina Faso.
Methodology: This study was a prospective cross-sectional study. It consisted of active surveillance of the safety of 3 anti-COVID-19 vaccines: Ad26.COV2. S vaccine, the BNT162b2 vaccine and the BBIBP-CorV vaccine. A total of 355 participants were included for each of the 3 vaccine types. These participants after informed consent received the vaccines and were monitored by telephone and/or home visit for 84 days.
Results: A total of 1329 participants were included, and between 77.2% and 100% of them were followed up for 84 days by vaccine type. The vast majority of adverse events were reported at Day1 after vaccination. Local adverse events were observed with the 3 types of vaccine. They were 11.6% (95% CI 9.1 ; 14.7), 4.9% (95% CI=3.3 ; 7.4) and 2.9% (95% CI 1.7 ; 5.2) respectively for Ad26.COV2. S Vaccine, BNT162b2 Vaccine and BBIBPCorV Vaccine respectively. Systemic adverse events were reported at 10% [95% CI 7.7 ; 13] for Ad26.COV2. S vaccine, 6.3% [95% CI 4.4 ; 8.9] for BNT162b2 vaccine and 3.5% [95% CI 2.0 ; 5.8] for BBIBP-CorV vaccine. With the BNT162b2 vaccine, women are 2.7 times likely to develop (95% CI = 1.1 - 6.5) more adverse events than men. No serious adverse events were reported.
Conclusion: The results obtained are similar to those of other approved vaccines used in routine immunization. The adverse events reported were transient. Adverse events were more frequently reported in women than in men, and generally lasted one to two days after vaccine administration. The essential limitation of this study was self-reporting by participants. The data were used on a daily basis to increase awareness and uptake of COVID-19 vaccination. The probability of occurrence and type of adverse events were key messages at follow-up.
Keywords
COVID-19 vaccine; Adverse events; Burkina Faso; SARSCoV- 2; China
COVID-19 vaccine articles; Adverse events articles; Burkina Faso articles; SARS-CoV-2 articles; China articles
Article Details
1. Introduction
Coronavirus 2019 or COVID-19 disease caused by SARS-CoV-2 first appeared in Wuhan, China [1,2]. The disease soon spread worldwide, creating a pandemic [3]. The disease has affected 491,576,851 people worldwide, causing 6,153,616 deaths by April 2022 [4].
In Africa, there were 8,580,381 confirmed cases at the end of March 2022 [5]. Burkina Faso had 20,858 confirmed cases of COVID-19 and 382 deaths in April 2022 [4].
Several measures have been undertaken to fight against the pandemic. To date, the development and administration of vaccines [6,7] is the most effective means of preventing the disease and its consequences [8].
Several types of vaccine have been developed worldwide. As of June 02, 2021, Burkina Faso has introduced vaccination into its control arsenal with a viral vector vaccine (Adenovirus vaccine). It was the COVID-19 Vaccine AstraZeneca (AZD1222) from the AstraZeneca + Oxford University laboratory. To date, five (05) other vaccines have been added. These are two mRNA vaccines: Comirnaty (BNT162b2) from BioNTech + Fosun Pharma; Jiangsu Provincial Center for Disease Prevention and Control (CDC) + Pfizer (Pfizer-BioNTech) and Moderna COVID-19 Vaccine (mRNA1273) from Moderna + National Institute of Allergy and Infectious Diseases (Moderna).
Burkina Faso uses a second viral vector vaccine: Ad26.COV2. S from the Janssen Pharmaceutica(J&J) laboratory. Finally, two inactivated vaccines are also used. These are the BBIBP-CorV vaccine from Sinopharm + Beijing Institute of Biological Products, and the CoronaVac vaccine from Sinovac Life Sciences, Beijing, China [9].
Burkina Faso has authorized, following a proposal from the National Immunization Technical Advisory Groups (NITAG), the vaccination of people aged 18 and over [10].
Several vaccination campaigns have been organized, reaching 5.85% of the population as of March 2022 [4]. Like other African countries, there are many hesitations, rumors and anti-vaccination campaigns against COVID-19.
Although safe and effective, vaccines can carry risks of adverse events that may or may not be causally related to vaccine use [11].
During the clinical phase adverse events were detected for all vaccines [9]. All COVID-19 vaccines have been introduced under Emergency Use Listing (EUL) and to date there is little data on adverse events related to vaccines [12], particularly in developing countries.
The aim of this active surveillance study was to produce a database of adverse events for Covid-19 vaccines in Burkina Faso. The results of this study will constitute new information on the safety profile of vaccines in the African context. They will also contribute to strengthen the case for vaccination against COVID-19 and to enrich the mapping of risk data.
Materials and Methods
2.1 Type of study
This was a prospective cross-sectional study. It consisted of active surveillance of the safety of COVID-19 vaccines.
2.2 Study population
This study concerned people vaccinated against Covid-19 with one of the three vaccines used in Burkina Faso (Ad26.COV2. S vaccine, BNT162b2 vaccine and BBIBP-CorV vaccine) and residing in the health regions of the study.
2.3 Inclusion criteria
- ✓ Be 18 years and above;
- ✓ Having received at Day 0 one of the following vaccines: Ad26.COV2. S vaccine ; BNT162b2 vaccine and BBIBP-CorV vaccine;
- ✓ Reside in the study regions during follow-up period;
- ✓ Agree to participate in the study and sign the consent form.
2.4 Non-inclusion criteria
Vaccinated individuals residing in the study health regions but planning to travel during the follow-up period ;
Subjects who have previously received another COVID-19 vaccine in the last 3 months than the vaccine received on the day of inclusion.
2.5 Study setting and sites
This study took place in three regions of Burkina Faso : the Centre, Centre-West and Hauts Bassins regions.
Vaccination centers selected by the country were the surveillance sites. A total of 30 sites were identified, i.e. 10 sites per health region.
2.6 Sampling
In each study health region, the most frequently used fixed vaccination sites were selected for the study. At each site, participants were included after having received at least one dose of one of the study vaccines. All vaccinated individuals meeting the study criteria were approached and consent was sought for inclusion.
Inclusion took place during routine vaccination periods and during vaccination campaigns. Sample size is calculated for each vaccine type using the Schwartz formula:
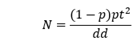
Referring to studies in other parts of the world, the prevalence of AEFI varies from 50% to 90% [13-18]. Given these prevalences, combined with administrative data from Burkina Faso, we decided to use a prevalence of 70% to calculate this study sample size.
With 95% confidence, we obtain a sample size of 323 participants for each type of vaccine in the study. Considering a 10% non-response rate, the sample size was at least 355 for each type of vaccine studied.
2.7 Data collection
A semi-structured questionnaire was designed to collect data. The questionnaire was tested before administration to the participants. The questionnaire was developed and adapted according to the adverse events for each type of COVID-19 vaccine during the clinical phases. Data were collected using the ODK (Open Data Kit) electronic tool and transferred to a server. Interviewers were recruited from among health workers at each vaccination site and trained in methodology and data collection.
Socio-demographic, medical, therapeutic and vaccination data were collected on the first day of inclusion.
Data on adverse events were collected throughout the follow-up period. Participants were contacted periodically to verify the occurrence of adverse events. In case a participant reports an adverse event, he or she was given the opportunity to immediately contact the study team at the vaccination site to report it.
Data collection on adverse events among study participants began during the post-vaccination observation period at vaccination site, then by daily and weekly phone calls. If a participant visited a medical center for adverse events during post-vaccination follow-up, data were completed through the medical record or the health department's consultation register.
2.8 Participant tracking
Each participant was followed for 3 months after the first dose of COVID-19 vaccine. If a second dose was administered within 3 months of the first dose, the subject was followed up until 3 months after the second dose. A follow-up period of 3 months was chosen, as it includes the time corresponding to the most common period of risk for Special Interest Adverse Events (SIAEs), and a similar duration to be used as a control period. Adverse event with particular interest leading to hospitalization were identified among diagnoses reported by the subject (or close relative) during follow-up. The list of Special Interest Adverse Interest (SIAI) is taken from the WHO manual on "Cohort Event Monitoring (CEM) for safety signal detection after vaccination with COVID-19 vaccines" [19].
Adverse event monitoring schedule
- ✓ Day 0 : completion of consent form, identification form and vaccination form
- ✓ Day 1 to Day 7 : daily completion of follow-up form;
- ✓ Day 7 to Day 28 : weekly completion of follow-up form;
- ✓ From Day 28 onwards, monthly completion of follow-up form;
- ✓ After a seconde dose of vaccine, the form is filled in weekly until Day 28 and monthly until Day 86.
Apart from Day0, other visits were made by phone or at home visit.
Any participant who was followed up for 3 months after the last dose of COVID-19 vaccine was considered to have completed the study.
Case management of adverse events
In the event of a case of adverse event of particular interest requiring management, participants were introduced into the national circuit for this management in accordance with the national procedure in force.
In Burkina Faso, Adverse Event Following Immunization (AEFIs) are managed by the National Agency for Pharmaceutical Regulation (NAPR). During all phases of this study, all the data identified for the study were simultaneously entered into the national tools and followed the national of notification circuit in Burkina Faso.
2.9 Data processing
Data collected was extracted on an Excel spreadsheet and processed prior to statistical analysis. Descriptive statistics were performed to present socio-demographic variables, medical history variables (chronic diseases, medical treatments, COVID-19 infection...), vaccination data (vaccine type and doses) and adverse event data (occurrence and duration of adverse events), using frequencies (n), percentages (%) and measures of central tendency such as mean (μ) and standard deviation (SD).
Statistical inference tests were carried out to assess the association between the occurrence of adverse events and socio-demographic data, medical history as well as the type of vaccine received, time to onset of events and duration of course. These inferences were performed using the Chi-square (χ 2) test, Fisher's exact test and Student's t-test. All these inferential tests use the assumption of a 95% confidence interval (CI) and a significance level of (p) < 0.05.
Statistical Package for the Social Sciences (SPSS) version 26.0 and Epi Info 7.1 were used for descriptive analyses and statistical tests.
2.10 Ethical considerations
This study was conducted in accordance with the International Ethical Guidelines for Health-Related Research Involving Humans published by the Council for International Organizations of Medical Sciences (CIOMS), the Declaration of Helsinki, Good Epidemiological Practice (GEP) and all applicable national laws and guidelines: law. n° 010-20:04/fan on the protection of personal data, decree n°2002-536/PRES/PM/MS/MESSRS creating an ethics committee for health research in Burkina Faso.
The study used self-reported data and data collected as part of healthcare provision where necessary. Data protection and confidentiality regulations were strictly adhered to during the collection, transmission, processing and storage of participant data.
Approval from the Health Research Ethics Committee (HREC) was obtained prior to the start of the study. Administrative authorization was also obtained from the Ministry of Health.
Written informed consent was requested from all subjects. Subjects had the right to refuse to participate or to withdraw from the study at any time and for any reason without prejudice. Serious cases were managed according to national procedures.
Results
3.1 Overall results
A total of 1473 people were screened. Of these, 1329 participants who met the inclusion criteria consented to take part in the study. After inclusion, participants were followed up on specific dates. At some specific follow-up points, some participants were missed.
The Table 1 below shows the distribution of people vaccinated and followed-up at specific checkpoints according to vaccine type.
|
Vaccine |
Vaccin Ad26.COV2. S |
Vaccin BNT162b2 |
Vaccin BBIBP-CorV |
|
Including |
508 |
446 |
375 |
|
J0 |
508 |
446 |
375 |
|
J1 |
463 |
338 |
309 |
|
J2 |
465 |
401 |
333 |
|
J3 |
446 |
371 |
338 |
|
J4 |
447 |
395 |
322 |
|
J5 |
449 |
382 |
319 |
|
J6 |
424 |
380 |
323 |
|
J7 |
442 |
360 |
333 |
|
J14 |
428 |
358 |
263 |
|
J28 |
418 |
322 |
286 |
|
J56 |
461 |
367 |
348 |
|
J84 |
458 |
363 |
344 |
Table 1: Distribution of people vaccinated and followed-up at different checkpoints, by vaccine type.
For the two-dose vaccines, 46.5% (IC95% 41.5-51.5%) of subjects received a second dose after the BNT162b2 vaccine, and 39.1% (IC95% 34.2-44.4%) after the BBIBP-Corv vaccine.
3.2 Socio-demographic characteristics of participants
The Table 2 below shows the socio-demographic characteristics of participants by vaccine type.
|
Socio demographic characteristics |
Vaccin Ad26.COV2. S |
Vaccin BNT162b2 |
Vaccin BBIBP-CorV |
|||||||||||
|
Age |
n |
% |
IC 95% |
n |
% |
IC 95% |
n |
% |
IC 95% |
|||||
|
18 to 29 years |
239 |
47,1 |
42,8 |
51,4 |
231 |
51,8 |
47,2 |
56,4 |
165 |
44 |
39,1 |
49,1 |
||
|
30 to 44 years |
144 |
28,4 |
24,6 |
32,4 |
143 |
32,1 |
27,9 |
36,5 |
116 |
30,9 |
26,5 |
35,8 |
||
|
45 years and more |
125 |
24,6 |
21,1 |
28,5 |
72 |
16,1 |
13 |
19,9 |
94 |
25,1 |
21 |
29,7 |
||
|
Gender |
||||||||||||||
|
Male |
191 |
37,6 |
33,5 |
41,9 |
118 |
26,5 |
22,6 |
30,7 |
127 |
33,9 |
29,3 |
38,8 |
||
|
Female |
317 |
62,4 |
58,1 |
66,5 |
328 |
73,5 |
69,3 |
77,4 |
248 |
66,1 |
61,2 |
70,7 |
||
|
Pregnancy |
||||||||||||||
|
Not pregnant |
304 |
95,9 |
93,1 |
97,6 |
279 |
85,9 |
81,6 |
89,2 |
246 |
100 |
0 |
0 |
||
|
Pregnant women |
13 |
4,1 |
2,4 |
6,9 |
46 |
14,2 |
10,8 |
18,4 |
0 |
0 |
0 |
0 |
||
|
Brest feeding |
||||||||||||||
|
Brest feeding women |
198 |
62,7 |
57,2 |
67,8 |
195 |
60,2 |
54,8 |
65,4 |
211 |
85,1 |
80 |
89,3 |
||
|
No brest feeding women |
118 |
37,3 |
32,2 |
42,8 |
129 |
39,8 |
34,6 |
45,2 |
37 |
14,9 |
10,7 |
20 |
||
|
Profession |
||||||||||||||
|
Student |
108 |
21,9 |
18,5 |
25,8 |
70 |
16 |
12,9 |
19,8 |
80 |
21,7 |
17,8 |
26,2 |
||
|
Retired person |
8 |
1,6 |
0,8 |
3,2 |
1 |
0,2 |
0 |
1,3 |
5 |
1,4 |
0,6 |
3,1 |
||
|
Public/private employee |
37 |
7,5 |
5,5 |
10,2 |
21 |
4,8 |
3,2 |
7,2 |
10 |
2,7 |
1,5 |
4,9 |
||
|
Retailer |
75 |
15,2 |
12,3 |
18,7 |
46 |
10,5 |
8 |
13,8 |
21 |
5,7 |
3,8 |
8,6 |
||
|
Farmer / Fisherman / breeder |
47 |
9,5 |
7,3 |
12,5 |
37 |
8,5 |
6,2 |
11,5 |
42 |
11,4 |
8,6 |
15,1 |
||
|
Smalls trades/Casual labourer |
59 |
12 |
9,4 |
15,1 |
31 |
7,1 |
5 |
9,9 |
34 |
9,2 |
6,7 |
12,6 |
||
|
Housewife |
152 |
30,8 |
26,9 |
35 |
209 |
47,8 |
43,2 |
52,5 |
161 |
43,8 |
38,8 |
48,9 |
||
|
Other to specify |
5 |
1 |
0,4 |
2,4 |
14 |
3,2 |
1,9 |
5,3 |
8 |
2,2 |
1,1 |
4,2 |
||
|
None |
2 |
0,4 |
0,1 |
1,5 |
8 |
1,8 |
0,9 |
3,6 |
7 |
1,9 |
0,9 |
3,9 |
||
|
Education level |
||||||||||||||
|
None |
163 |
32,1 |
28,2 |
36,3 |
144 |
32,3 |
28,1 |
36,7 |
166 |
44,3 |
39,3 |
49,3 |
||
|
Primary |
107 |
21,1 |
17,7 |
24,8 |
149 |
33,4 |
29,2 |
37,9 |
89 |
23,7 |
19,7 |
28,3 |
||
|
Post-primary |
10 |
2 |
1,1 |
3,6 |
15 |
3,4 |
2,1 |
5,5 |
11 |
2,9 |
1,7 |
5,2 |
||
|
Secondary |
165 |
32,5 |
28,6 |
36,7 |
99 |
22,2 |
18,6 |
26,3 |
82 |
21,9 |
18 |
26,3 |
||
|
Senior |
63 |
12,4 |
9,8 |
15,6 |
39 |
8,7 |
6,5 |
11,7 |
27 |
7,2 |
5 |
10,3 |
||
Table 2: Socio-demographic characteristics of participants.
3.3 Medical and therapeutic history of participants
The Table 3 below shows the subjects' medical and therapeutic history at the time of vaccination.
|
Medical and therapeutic history |
Vaccin Ad26.COV2. S |
Vaccin BNT162b2 |
Vaccin BBIBP-CorV |
||||||||||
|
n |
% |
IC 95% |
n |
% |
IC 95% |
n |
% |
IC 95% |
|||||
|
Medical history |
|||||||||||||
|
Heart disease |
1 |
0,2 |
0,0 |
1,1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
HTA |
15 |
3,0 |
1,8 |
4,8 |
7 |
1,6 |
0,8 |
3,2 |
3 |
0,8 |
0,3 |
2,3 |
|
|
Asthma |
5 |
1,0 |
0,4 |
2,3 |
0 |
0 |
0 |
0 |
2 |
0,5 |
0,2 |
1,9 |
|
|
Diabetes |
3 |
0,6 |
0,2 |
1,7 |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
Sickle cell disease |
3 |
0,6 |
0,2 |
1,7 |
3 |
0,7 |
0,2 |
2,0 |
2 |
0,5 |
0,2 |
1,9 |
|
|
Chronic lung disease |
1 |
0,2 |
0,0 |
1,1 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0 |
0 |
0 |
|
|
Neuropathy |
1 |
0,2 |
0,0 |
1,1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
COVID-19 |
4 |
0,8 |
0,3 |
2,0 |
1 |
0,2 |
0,0 |
1,3 |
1 |
0,3 |
0,1 |
1,5 |
|
|
Nephropathy |
1 |
0,2 |
0,0 |
1,1 |
0 |
0 |
0 |
0 |
0 |
0 |
|||
|
Hepatopathy |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0,3 |
0,1 |
1,5 |
|
|
Cancer |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0,3 |
0,1 |
1,5 |
|
|
HIV |
0 |
0 |
0 |
0 |
1 |
0,2 |
0,0 |
1,3 |
2 |
0,5 |
0,2 |
1,9 |
|
|
Arthralgia |
2 |
0,4 |
0,1 |
1,4 |
2 |
0,5 |
0,1 |
1,6 |
2 |
0,5 |
0,2 |
1,9 |
|
|
Other medical history |
|||||||||||||
|
Gastro-duodenal ulcers |
2 |
0,4 |
0,1 |
1,4 |
5 |
1,1 |
0,5 |
2,6 |
0 |
0 |
0 |
0 |
|
|
Sinusitis |
1 |
0,2 |
0,0 |
1,1 |
2 |
0,5 |
0,1 |
1,6 |
0 |
0 |
0 |
0 |
|
|
Treatment history |
|||||||||||||
|
Antihypertensives |
7 |
1,4 |
0,7 |
2,8 |
4 |
0,9 |
0,4 |
2,3 |
1 |
0,3 |
0,1 |
1,5 |
|
|
Antidiabetics |
2 |
0,4 |
0,1 |
1,4 |
0 |
0,0 |
0 |
0 |
0 |
0,0 |
0 |
0 |
|
|
Non-steroidal antiinflamatory drugs |
0 |
0 |
0 |
0 |
0 |
0,0 |
0 |
0 |
0 |
0,0 |
0 |
0 |
|
|
Steroidal antiinflamatory drugs |
0 |
0 |
0 |
0 |
0 |
0,0 |
0 |
0 |
1 |
0,3 |
0,1 |
1,5 |
|
|
ARV |
0 |
0 |
0 |
0 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0 |
0 |
0 |
|
|
Pill |
1 |
0,2 |
0,0 |
1,1 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0 |
0 |
0 |
|
Table 3: Participants' medical and therapeutic history.
3.4 Reported adverse events
Adverse events were reported from the very first day of vaccination.
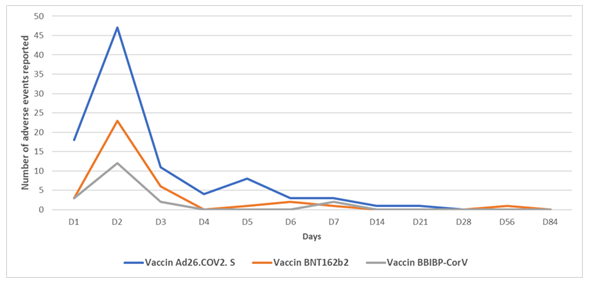
For all 3 types of vaccine studied, the vast majority of events were reported one day after vaccination. From day 1 onwards, fewer and fewer events were reported, disappearing completely by day 84 post-vaccination. On day 5, a rebound of events was observed.
The curve below shows the evolution of reported events over time.
3.5 Type of adverse events reported
Local and systemic adverse events were reported. They were mainly observed during the first week after administration.
The Table 4 below shows the frequencies of adverse events types studied according to the study vaccines .
|
Adverse events types |
Vaccin Ad26.COV2. S |
Vaccin BNT162b2 |
Vaccin BBIBP-CorV |
|||||||||
|
n |
% |
IC 95% |
n |
% |
IC 95% |
n |
% |
IC 95% |
||||
|
Local adverse events |
59 |
11,6 |
9,1 |
14,7 |
22 |
4,9 |
3,3 |
7,4 |
11 |
2,9 |
1,7 |
5,2 |
|
Systemic adverse events |
51 |
10,0 |
7,7 |
13,0 |
28 |
6,3 |
4,4 |
8,9 |
13 |
3,5 |
2,0 |
5,8 |
Table 4: Frequency of adverse event types by vaccine type.
From the second week (Day 14), only 4 events were reported. These were essentially a local adverse event of arm weakness at Day 14 attributable to the Ad26.COV2. S vaccine, 2 systemic headache-type adverse events reported at Day 21 and Day 28 for the same vaccine, and a headache-type signal at Day 56 for the BNT162b2 vaccine.
The Table 5 below shows the events reported by vaccine type during 84 days of follow-up.
|
Adverse events |
Vaccin Ad26.COV2. S |
Vaccin BNT162b2 |
Vaccin BBIBP-CorV |
|||||||||
|
n |
% |
IC 95% |
n |
% |
IC 95% |
n |
% |
IC 95% |
||||
|
Local adverse events |
||||||||||||
|
Site pain |
50 |
9,8 |
7,6 |
12,7 |
16 |
3,6 |
2,2 |
5,8 |
10 |
2,7 |
1,5 |
4,8 |
|
Swelling of the site |
1 |
0,2 |
0,0 |
1,1 |
4 |
0,9 |
0,4 |
2,3 |
1 |
0,3 |
0,1 |
1,5 |
|
Site redness |
7 |
1,4 |
1,2 |
3,8 |
1 |
0,2 |
0,0 |
1,3 |
1 |
0,3 |
0,1 |
1,5 |
|
Site warmth |
0 |
0,0 |
2 |
0,5 |
0,1 |
1,6 |
2 |
0,5 |
0,2 |
1,9 |
||
|
Adenopathy |
0 |
0,0 |
2 |
0,5 |
0,1 |
1,6 |
0 |
0,0 |
||||
|
Ecchymosis |
0 |
0,0 |
2 |
0,5 |
0,1 |
1,6 |
0 |
0,0 |
||||
|
Site rash |
5 |
1,0 |
0,4 |
2,3 |
0 |
0,0 |
0 |
0,0 |
||||
|
Arm stiffness |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
0 |
0,0 |
||||
|
Induration of site |
1 |
0,2 |
0,0 |
1,1 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0,0 |
||
|
Arm weakness |
6 |
1,2 |
0,5 |
2,6 |
4 |
0,9 |
0,4 |
2,3 |
0 |
0,0 |
||
|
Pruritus |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
0 |
0,0 |
||||
|
Arm pain |
5 |
1,0 |
0,4 |
2,3 |
4 |
0,9 |
0,4 |
2,3 |
1 |
0,3 |
0,1 |
1,5 |
|
Finger cramps |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
0 |
0,0 |
||||
|
Neck pain |
0 |
0,0 |
1 |
0,2 |
0,0 |
1,3 |
1 |
0,3 |
0,1 |
1,5 |
||
|
Arm heaviness |
0 |
0,0 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0,0 |
||||
|
Systemic adverse events |
||||||||||||
|
Headache |
27 |
5,3 |
3,7 |
7,6 |
14 |
3,1 |
1,9 |
5,2 |
12 |
3,2 |
1,8 |
5,5 |
|
Malaise |
15 |
3,0 |
1,8 |
4,8 |
8 |
1,8 |
0,9 |
3,5 |
0 |
0,0 |
||
|
Fever |
16 |
3,2 |
2,0 |
5,1 |
13 |
2,9 |
1,7 |
4,9 |
4 |
1,1 |
0,4 |
2,7 |
|
Fatigue / Asthenia |
21 |
4,1 |
2,7 |
6,2 |
9 |
2,0 |
1,1 |
3,8 |
1 |
0,3 |
0,1 |
1,5 |
|
Chills |
7 |
1,4 |
0,7 |
2,8 |
0 |
0,0 |
0 |
0,0 |
||||
|
Arthralgia |
17 |
3,4 |
2,1 |
5,3 |
3 |
0,7 |
0,2 |
2,0 |
2 |
0,5 |
0,2 |
1,9 |
|
Myalgia |
19 |
3,7 |
2,4 |
5,8 |
2 |
0,5 |
0,1 |
1,6 |
4 |
1,1 |
0,4 |
2,7 |
|
Nausea/ Vomiting |
5 |
1,0 |
0,4 |
2,3 |
4 |
0,9 |
0,4 |
2,3 |
0 |
0,0 |
||
|
Diarrhea |
3 |
0,6 |
0,2 |
1,7 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0,0 |
||
|
Respiratory disorders |
0 |
0,0 |
0 |
0,0 |
0 |
0,0 |
||||||
|
Dizziness |
8 |
1,6 |
0,8 |
3,1 |
4 |
0,9 |
0,4 |
2,3 |
0 |
0,0 |
||
|
Urticaria |
4 |
0,8 |
0,3 |
2,0 |
0 |
0,0 |
0 |
0,0 |
||||
|
Multiple lymph nodes |
0 |
0,0 |
0 |
0,0 |
0 |
0,0 |
||||||
|
Facial swelling |
0 |
0,0 |
0 |
0,0 |
0 |
0,0 |
||||||
|
Paresthesia |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
0 |
0,0 |
||||
|
Facial paralysis |
0 |
0,0 |
0 |
0,0 |
0 |
0,0 |
||||||
|
Insomnia |
2 |
0,4 |
0,1 |
1,4 |
1 |
0,2 |
0,0 |
1,3 |
0 |
0,0 |
||
|
Muscle weakness |
2 |
0,4 |
0,1 |
1,4 |
2 |
0,5 |
0,1 |
1,6 |
0 |
0,0 |
||
|
Tinnitus |
0 |
0,0 |
0 |
0,0 |
0 |
0,0 |
||||||
|
Courbatures |
0 |
0,0 |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
||||
|
Chest pain |
1 |
0,2 |
0,0 |
1,1 |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
||
|
Eye pain |
1 |
0,2 |
0,0 |
1,1 |
0 |
0,0 |
0 |
0,0 |
||||
|
Cold |
1 |
0,2 |
0,0 |
1,1 |
2 |
0,5 |
0,1 |
1,6 |
0 |
0,0 |
||
Table 5: Adverse events reported by vaccine type.
3.6 Factors associated with the occurrence of adverse events.
The occurrence of adverse events was not correlated with vaccine type, age or medical history. Pregnancy, breast-feeding or history of SARSCoV-2 infection and the occurrence of adverse events showed no significant association. However, based on an analysis with well-defined population subgroups, we observed an association between gender and the occurrence of adverse events with the BNT162b2 vaccine. Indeed, with the mRNA vaccine, women are 2.7 times (95% CI = 1.1 - 6.5) likely to develop more adverse events than men.
The Table 6 below shows the factors associated with adverse events by vaccine type.
|
Variables |
Presence of adverse events |
Absence of adverse events |
OR |
IC 95% |
||
|
By gender and vaccine type |
||||||
|
Vaccin Ad26.COV2. S |
Male |
23 |
168 |
|||
|
Female |
53 |
264 |
3,5 |
2,0 |
6.1 |
|
|
Vaccin BNT162b2 |
Male |
15 |
103 |
|||
|
Female |
21 |
307 |
2,1 |
1,0 |
4,3 |
|
|
Vaccin BBIBP-CorV |
Male |
5 |
93 |
|||
|
Female |
7 |
204 |
1,6 |
0,4 |
5,1 |
|
|
By vaccine type |
||||||
|
Vaccin Ad26.COV2. S |
76 |
432 |
3,3 |
2,0 |
5,7 |
|
|
Vaccin BNT162b2 |
36 |
410 |
1,6 |
0,9 |
3,0 |
|
|
Vaccin BBIBP-CorV |
19 |
356 |
||||
Table 6: Factors associated with the occurrence of adverse events by vaccine type and gender.
4. Discussions
4.1 Participants characteristics
Mass vaccination against COVID-19 was a major opportunity to include the majority of participants. Indeed, over 95% of subjects were included on this occasion. Over 75.5% of those vaccinated were under 45 years of age. The national guidelines for vaccination against COVID-19 in Burkina Faso set people over 65 years of age as the highest priority. However, vaccination in Burkina Faso concerns all adults over of 18 years old. However, it has to be admitted that the national objective of vaccinating 70% of the total population, while focusing on the adult population and secondarily on 12- to 17-year-olds, was difficult to achieve. Given the age structure of Burkina Faso's population, over 90% of the general population is under 50 (RGPH 2019). This age group is the most active and dynamic. It is also the most involved in international travel. People in this age bracket would vaccinate to facilitate their international travel.
For all the three types of vaccine studied, women represent the most highly vaccinated population group. They represent between 61% and 73% of those vaccinated, depending on the type of vaccine. In Burkina Faso, women are the main users of health services. First, they use health services during gestation, then during delivery. Deliveries in health facilities account for 94% nationwide. In rural areas, they represent 93% according to EDS 2021 [20]. Later, mothers still have to go there for consultations on healthy infants and for vaccination. Health centers are increasingly becoming a social center for women, hence the name "social promotion center". As a result, they have become accustomed to them, and trust is growing between them and the health workers and mothers who work there.
Housewives, students and shopkeepers are the most vaccinated. This corroborates the above results. Administrative data from the Ministry of Health and Public Hygiene on vaccination against COVID-19 show similar patterns in terms of the demographic characteristics of those vaccinated, notably age and sex.
In this study, only 3.3% of participants have chronic diseases (hypertension, diabetes, sickle-cell anemia, cancer, nephropathy, neuropathy, asthma, etc.). In our context, there is a lack of awareness of chronic diseases among the population. Most people are unaware of the specific chronic pathologies they suffer from. Yet this group is the high-priority population recommended by the National Immunization Technical Group (NITAG-BF) and implemented by Burkina Faso's Ministry of Health and Public Hygiene. They have been a priority for vaccination since June 02, 2021, the launch date of COVID-19 vaccination in Burkina Faso
4.2 Mild manifestations
The safety profile during 84 days of follow-up was similar to that of other previously approved vaccines. The local adverse events reported were identical to those observed with the other vaccines in the vaccination program. These were essentially the frequent events commonly observed. Reactions were generally mild and of short duration. Kleyton Santos Mederos et al. [21] found the same signs at the injection site: pain, induration, redness or erythema, swelling, itching and muscle weakness [21].
From the second day after vaccination, adverse events generally diminished. They were considerably more frequent on day 1. On day 3, only four types of locally adverse events were reported: pain, swelling, ecchymosis and adenopathy. From day 4 to the end of follow-up, events were very rare or even absent. Most Phase II trials show that adverse events of mild to moderate severity are frequent and self-limiting, but less frequent in the elderly [22].
Several studies have found systemic adverse events similar to ours. In these studies, events were such as fever, febricula, hyperthermia, headache, fatigue, vomiting, diarrhea, muscle pain, joint pain, cough, nausea, dyspnea, appetite disorders, dizziness, mucous membrane abnormalities, pruritus, hypersensitivity, syncope, asthenia, rhinorrhea [23-26].
In our study, vaccinated people who reported more adverse events were women. Similar results were observed by Bouman A et al. [27]. We also note that with the BNT162b2 vaccine, women were 2.7 times more likely to present adverse events than men. These differences could be explained by biological effects between gender and immune responses [28]. Similar results were also obtained by Giancarlo Ripabelli et al. [29].
Individuals with a history of SARS-CoV-2 infection, numbering only 6 in this study, also showed only minor local effects. These effects were also of short duration.
There was no association between the occurrence of adverse events and a history of chronic disease. Since people with underlying illnesses were described as having a higher risk of serious consequences in the event of SARSCOv-2 contagion. They were given priority to receive the COVID-19 vaccine. Only 25 (1.9%) of the subjects included in our study with prior knowledge of hypertension were vaccinated against COVID-19, despite the fact that this condition is known to be one of the main co-morbid causes of death due to COVID-19 [30]. Rare manifestations not described in the literature were not identified in this study.
4.3 Serious events
No serious events were identified during follow-up. However, from Day 4 , eight (8) cases of hospitalization were reported. All hospitalized patients presented symptoms such as headache, fever, nausea and vomiting. For all these cases, a malaria rapid diagnostic test (RDT) was performed and malaria was confirmed.
This is explained by the fact that the inclusion and follow-up of these participants was carried out during the period of high malaria transmission. Inclusion of participants began on August 16, 2022. They were all treated and discharged from the health facilities. In Phase II clinical trials, serious adverse events were very rare [22]. In other studies prior to the vaccine roll-out phase for broad-spectrum vaccination, serious adverse events were identified in the form of death, hospitalization and thrombotic complications [26,31,32].
5. Conclusion
This study has shown that local adverse events were the most observed for all the vaccines against COVID-19 vaccines . They are similar to other approved vaccines used in routine EPI. Short-term adverse events are more frequent. Adverse events were more frequently reported in women than in men, and generally lasted one to two days after vaccine administration. No serious events were reported during 84 days of follow-up. The most important events notified were immediately reported to the health district level for management and reporting. The operational level also used these data to raise awareness and increase uptake of COVID-19 vaccines. The probability of occurrence and type of adverse events were key messages during follow-up.
However, there are limitations to this study. Firstly, it concerns self-reported data. In a general population, these declarations may contain shortcomings. However, they have the advantage of being collected without any constraints in a general population. Recall bias was minimized by daily and weekly calls. It was also not possible to establish a precise link between the occurrence of adverse events and others factors, such as a history of SARSCOV2 infection, due to the limited number of cases. This study reinforces the confidence of the general population in vaccination against COVID-19, and reduces the potential for vaccine hesitancy in our context.
References
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 579 (2020): 265-9.
- Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J 96 (2020): 753-8.
- Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis 20 (2020): e102-7.
- Johns Hopkins Coronavirus Resource Center [Internet]. [cité 29 avr 2022]. COVID-19 Map. Disponible sur: https://coronavirus.jhu.edu/map.html
- WHO Coronavirus (COVID-19) Dashboard [Internet]. 2022 avr. Disponible sur: https://covid19.who.int
- Haque A, Pant AB. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 8 (2020): 739.
- Kim KD, Hwang I, Ku KB, et al. Progress and Challenges in the Development of COVID-19 Vaccines and Current Understanding of SARS-CoV-2- Specific Immune Responses. J Microbiol Biotechnol 30 (2020): 1109-15.
- Li L, Guo P, Zhang X, et al. SARS-CoV-2 vaccine candidates in rapid development. Hum Vaccines Immunother 17 (2021): 644-53.
- Kaur RJ, Dutta S, Bhardwaj P, et al. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J Clin Biochem IJCB 36 (2021): 427-39.
- GTCV-BF. Rapport de l’atelier du groupe technique consultatif sur la vaccination du Burkina Faso (GTCV-BF) relatif à la deuxième révision des recommandations sur le vaccin contre la COVID-19 et l’introduction du vaccin contre l’hépatite B à la naissance. Loumbila Beach, Burkina Faso (2021).
- CDC COVID-19 Response Team, Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine - United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep 70 (2021): 46-51.
- Riad A, Schünemann H, Attia S, et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int J Environ Res Public Health 18 (2021): 7859.
- Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 21 (2021): 939-49.
- Ossato A, Tessari R, Trabucchi C, et al. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: a post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm Sci Pract ejhpharm (2021).
- Dziedzic A, Riad A, Attia S, et al. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J Clin Med 10 (2021): 5338.
- Klugar M, Riad A, Mekhemar M, et al. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 10 (2021): 752.
- Sultana A, Shahriar S, Tahsin MR, et al. A Retrospective Cross-Sectional Study Assessing Self-Reported Adverse Events following Immunization (AEFI) of the COVID-19 Vaccine in Bangladesh. Vaccines 9 (2021): 1090.
- Chakraborty A, Reval N, Kamath L. Adverse Events Following COVID-19 Vaccination in Selected Apartments in Bangalore, India. Cureus 14 (2022): e21809.
- World Health Organization. Protocol template to be used as a template for observational study protocols: Cohort event monitoring (cem) for safety signal detection after vaccination with covid-19 vaccines [Internet] (2021).
- INSD et ICF. Enquête Démographique et de Santé du Burkina Faso 2021. Ouagadougou, Burkina Faso et Rockville, Maryland, USA: INSD et ICF (2022).
- Medeiros KS, Costa APF, Sarmento ACA, et al. Side effects of COVID-19 vaccines: a systematic review and meta-analysis protocol of randomised trials. BMJ Open 12 (2022): e050278.
- Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 50 (2021): 279-83.
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet 395 (2020): 1845-54.
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Lond Engl 396 (2020): 479-88.
- Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet Lond Engl 396 (2020): 887-97.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet 396 (2020): 467-78.
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 11 (2005): 411-23.
- Cuschieri S, Borg M, Agius S, et al. Adverse reactions to Pfizer-BioNTech vaccination of healthcare workers at Malta’s state hospital. Int J Clin Pract 75 (2021): e14605.
- Ripabelli G, Tamburro M, Buccieri N, et al. Active Surveillance of Adverse Events in Healthcare Workers Recipients After Vaccination with COVID-19 BNT162b2 Vaccine (Pfizer-BioNTech, Comirnaty): A Cross-Sectional Study. J Community Health 47 (2022): 211-25.
- Characteristics of COVID-19 patients dying in Italy [Internet]. [cité 13 juin 2023]. Disponible sur: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths
- Piera Carbonell A, Frías Vargas M, García Vallejo O, et al. [COVID-19 and thromboprophylaxis: Recommendations for our clinical practice in Primary Care]. Semergen 46 (2020): 479-86.
- Porres-Aguilar M, Lazo-Langner A, Panduro A, et al. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: An emerging cause of splanchnic vein thrombosis. Ann Hepatol 23 (2021): 100356.
Related PubMed Articles
- Estimates of SARS-CoV-2 vaccine effectiveness against outpatient medically attended SARS-CoV-2 infection from April 2023 through August 2024 in Hong Kong: A test-negative design study.
- Synergistic antiviral activity of a cathepsin B/L inhibitor and a TMPRSS2 inhibitor against SARS-CoV-2 in vitro and in vivo.
- The XBB.1.5 COVID-19 vaccine elicits a durable antibody response to ancestral and XBB.1.5 SARS-CoV-2 spike proteins.
- Emerging lipid nanoparticle systems capable of efficient intramuscular RNA delivery.
- Immune counter-evolution: immortalized B cell clones can undergo ex vivo directed evolution to counteract viral escape.
- Potential of MRNA vaccines for mpox prevention: current evidence and future directions.
- De Novo Immune Induction After COVID-19 Vaccination Under B-Cell Depletion Is Characterized by Robust T-Cellular Immunity in Patients With Inflammatory Central Nervous System Disease.