Association of Glycemic Status with Clinico-Biochemical Parameters and Serum Tumor Marker Profiles in Adult Patients: A Cross-Sectional Study
Abdul Razzaq*,1, Mst Shahina Khatun2, Md Shafiul Azam3, Mohammad Abdus Salam4, Mst Papiya Sultana Popy5, Tamanna Khondokar Imu6, Bhazan Chandra Majumder7, Md. Mohibur Rahman8, Rashidul Islam Dip9, Md.Nahid Hasan10
1Digital Diagnostic Centre, Bhola, Bangladesh
2Royal Surrey County Hospital NHS Foundation Trust, Guildford, UK
3University of Technology Sydney, Sydney, Australia
4Bangladesh Medical University, Dhaka, Bangladesh
5Shaheed Tajuddin Ahmed Medical College Hospital, Gazipur, Bangladesh
6Dana Diagnostic & Consultation Center, Dhaka, Bangladesh
7New Dhaka Modern Clinic, Dhaka, Bangladesh
8Royal Surrey County Hospital NHS Foundation Trust, Guildford, UK.
9Gazi Medical College, Khulna, Bangladesh
10Amzad Diagnostic Center, Shrerpur, Bangladesh
*Corresponding author: Abdul Razzaq, Digital Diagnostic Centre, Bhola, Bangladesh.
Received: 05 January 2026; Accepted: 12 January 2026; Published: 23 January 2026
Article Information
Citation: Abdul Razzaq, Mst Shahina Khatun, Md Shafiul Azam, Mohammad Abdus Salam, Mst Papiya Sultana Popy, Tamanna Khondokar Imu, Bhazan Chandra Majumder, Md. Mohibur Rahman, Rashidul Islam Dip, Md.Nahid Hasan. Association of Glycemic Status with Clinico- Biochemical Parameters and Serum Tumor Marker Profiles in Adult Patients: A Cross-Sectional Study. International Journal of Applied Biology and Pharmaceutical Technology. 17 (2026): 01-09.
Share at FacebookAbstract
Background: Altered glycemic status is associated with metabolic disturbances that may influence routine biochemical parameters and serum tumor marker levels, potentially complicating clinical interpretation.
Objective: This study aimed to evaluate the association of glycemic status with clinico-biochemical parameters and serum tumor marker profiles in adult patients, with particular emphasis on sex-based differences and abnormality frequencies.
Methods: In this cross-sectional study, adult patients were evaluated for demographic characteristics, biochemical parameters, and serum tumor markers. Biochemical variables were compared between males and females and Tumor marker levels were summarized using descriptive statistics. Abnormal biochemical and tumor marker frequencies were calculated based on standard reference ranges and expressed as percentages. Correlation analysis and multivariable regression models were performed to assess associations between biochemical parameters and tumor markers.
Results: A total of 181 patients (126 males, 55 females; mean age 55.04 ± 16.51 years) were analyzed. Significant sex-based differences were observed for HbA1c (8.69 ± 2.14 vs 7.96 ± 1.79%, p = 0.020), serum urea (42.89 ± 28.99 vs 65.45 ± 64.79 mg/dL, p = 0.017), AST (30.91 ± 26.44 vs 44.69 ± 46.19 U/L, p = 0.044), total cholesterol (164.36 ± 48.94 vs 188.11 ± 57.85 mg/dL, p = 0.001), and LDL cholesterol (100.37 ± 37.98 vs 115.28 ± 37.92 mg/dL, p = 0.018). Tumor marker levels showed marked variability, particularly AFP (17.08 ± 119.21 ng/mL), CA-125 (32.26 ± 91.61 U/mL), and CA15-3 (11.9 ± 108.36 U/mL), whereas CEA (2.65 ± 4.49 ng/mL) and PSA (1.73 ± 3.55 ng/mL) were more stable. Abnormal AST (25.45%), CEA (27.27%), AFP (41.82%), and CA-125 (21.82%) were more frequent among females, while abnormal creatinine (29.37%) and PSA (56.35%) were observed exclusively in males. Multivariable regression revealed consistent positive associations between serum magnesium and CEA (β = 6.93, p < 0.001), AFP (β = 90.72, p = 0.036), CA19-9 (β = 82.72, p < 0.001), CA-125 (β = 93.69, p = 0.012), and CA15-3 (β = 70.27, p = 0.001), whereas serum calcium showed significant inverse associations with AFP (β = −238.47, p < 0.001), CA19-9 (β = −74.77, p = 0.005), and CA15-3 (β = −58.44, p = 0.013). Age was independently associated with CEA (p = 0.040) and PSA (p < 0.001).
Conclusion: Glycemic-associated metabolic alterations are linked to selective biochemical abnormalities and variations in serum tumor marker profiles, with notable gender-based differences.
Keywords
Glycemic status, Biochemical parameters, Tumor markers, Sex differences, Cross-sectional study
Article Details
Introduction
Glycemic dysregulation, encompassing prediabetes and diabetes mellitus, represents a major global public health challenge and is closely linked to a wide spectrum of metabolic and systemic abnormalities. Chronic hyperglycemia induces complex pathophysiological changes involving insulin resistance, oxidative stress, low-grade inflammation, and altered lipid and protein metabolism1. These metabolic disturbances not only contribute to classical complications such as cardiovascular, hepatic, and renal dysfunction but may also influence routine biochemical parameters commonly used in clinical practice2.
In recent years, increasing attention has been directed toward the interaction between glycemic status and serum tumor markers. Tumor markers such as carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), cancer antigen-125 (CA-125), cancer antigen-15-3 (CA 15-3), cancer antigen-19-9 (CA 19-9), and prostate-specific antigen (PSA) are widely utilized in oncology for disease screening, prognosis, and treatment monitoring3,4. However, elevations in these markers are not exclusively associated with malignancy and may occur in a variety of benign conditions, including metabolic disorders, chronic inflammation, liver disease, and renal impairment. Consequently, altered glycemic states may complicate the clinical interpretation of tumor marker results, potentially leading to diagnostic ambiguity5.
Several studies have reported associations between diabetes and elevated levels of specific tumor markers, suggesting that hyperglycemia-related metabolic stress, hepatic dysfunction, and insulin resistance may modulate tumor marker expression or clearance6,7. Additionally, gender-specific physiological differences in hormonal profiles, body composition, and metabolic regulation may further influence both biochemical parameters and tumor marker concentrations. Despite these observations, existing evidence remains inconsistent, and comprehensive evaluations integrating glycemic status, clinico-biochemical parameters, tumor marker profiles, and gender-based differences are limited, particularly in adult populations from resource-constrained settings8,9.
Understanding these associations is clinically relevant, as misinterpretation of tumor marker elevations in metabolically dysregulated patients may result in unnecessary investigations, patient anxiety, and increased healthcare burden10. A systematic assessment of biochemical abnormalities and tumor marker variations across different glycemic states can provide valuable insight into non-malignant influences on tumor marker levels and support more accurate clinical decision-making11. The present study aimed to evaluate the association between glycemic status and clinico-biochemical parameters alongside serum tumor marker profiles in adult patients.
Materials and Methods
Study Design and Setting
This analytical cross-sectional study was conducted among adult patients attending Dhaka Cancer Hospital, Dhaka, Bangladesh, over a four-month period from June 2024 to September 2024. The study was designed to investigate the association between glycemic status, clinico-biochemical parameters, and serum tumor marker profiles, with particular emphasis on sex-based differences, abnormality patterns, and independent multivariable associations.
Study Population and Sample Size
A total of 181 adult participants aged 18 years and above were consecutively enrolled during the study period. Participants were included if complete data on glycemic indices, biochemical parameters, and serum tumor markers were available. Individuals with missing laboratory values or incomplete clinical records were excluded to ensure analytical robustness. The final study population comprised 126 males and 55 females, allowing for reliable sex-stratified analyses.
Classification of Glycemic Status
Participants were classified into normoglycemic, prediabetic, and diabetic groups according to internationally accepted diagnostic criteria. Glycemic status was determined using fasting blood glucose, two-hour postprandial blood glucose, glycated hemoglobin (HbA1c), and oral glucose tolerance test results where available. Consistent diagnostic cut-off values were applied across all participants to minimize misclassification and ensure comparability between glycemic categories.
Data Collection and Clinical Assessment
Demographic and clinical information, including age, sex, marital status, and relevant medical history, was collected using a structured data collection form. Venous blood samples were obtained from all participants after an overnight fast, following standard phlebotomy techniques and routine hospital laboratory protocols.
Biochemical Analysis
All biochemical measurements were performed using fully automated clinical chemistry analyzers in accordance with manufacturer-recommended procedures and internal quality control standards. The analyzed parameters included glycemic indices (fasting plasma glucose and HbA1c), lipid profile parameters (total cholesterol, triglycerides, LDL-cholesterol, and HDL-cholesterol), liver function tests (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and serum albumin), renal function tests (serum creatinine and blood urea), and electrolyte and mineral levels (sodium, potassium, calcium, and magnesium). All results were interpreted using standard reference ranges routinely applied in clinical practice.
Serum Tumor Marker Analysis
Serum concentrations of carcinoembryonic antigen, prostate-specific antigen, cancer antigen-125, cancer antigen-19-9, cancer antigen-15-3, and alpha-fetoprotein were measured using standardized immunoassay techniques. Manufacturer-recommended reference ranges were used to classify tumor marker values as normal or abnormal. Tumor markers were analyzed both as continuous variables and as categorical variables to facilitate descriptive and inferential analyses.
Definition of Abnormal Biochemical and Tumor Marker Values
Abnormal biochemical and tumor marker values were defined based on established laboratory reference intervals. Sex-specific interpretation was applied where biologically appropriate, particularly for prostate-specific antigen. The frequencies of abnormal biochemical and tumor marker values were calculated separately for male and female participants to assess sex-based disparities.
Statistical Analysis
Data were analyzed using statistical software R (RStudio 2024.12.0). Continuous variables were expressed as mean ± SD and categorical variables as frequencies and percentages. Correlation and multivariable regression analyses were conducted to assess associations between glycemic status and clinico-biochemical parameters. A p-value <0.05 was considered statistically significant.
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment, and participant confidentiality was strictly maintained throughout the study.
Results
Demographic Characteristics:
A total of 181 adult patients were included in the analysis, comprising 126 males and 55 females. The overall mean age was 55.04 ± 16.51 years; mean age was 54.66 ± 15.53 years among males and 55.93 ± 18.54 years among females, with no statistically significant difference between gender (p > 0.05). Demographic characteristics were comparable between male and female participants, as presented in Table 1. The majority of patients belonged to the 40–59 year age group, and 91.16% of the study population were married.
Table 1: Demographic Characteristics of the Patients
|
Age group |
Frequency |
Marital status |
||||
|
Male |
Female |
Married |
Unmarried |
|||
|
Male |
Female |
Male |
Female |
|||
|
20-39 |
22 |
11 |
12 |
5 |
10 |
6 |
|
40-59 |
56 |
22 |
56 |
22 |
0 |
0 |
|
60-79 |
40 |
16 |
40 |
16 |
0 |
0 |
|
80-100 |
8 |
6 |
8 |
4 |
0 |
2 |
|
Total |
126 |
55 |
116 |
49 |
10 |
6 |
Biochemical Parameters
Comparison of biochemical parameters between male and female participants demonstrated that only a limited number of variables differed significantly between sexes. Specifically, HbA1c (%), serum urea, AST, total cholesterol, and LDL cholesterol showed statistically significant differences (p < 0.05), whereas all other biochemical parameters did not vary significantly between male and female participants, as presented in Table 2. Female participants exhibited higher mean values of two-hour postprandial blood glucose, serum creatinine, urea, bilirubin, ALT, ALP, HDL cholesterol, LDL cholesterol, triglycerides, sodium, magnesium, and calcium compared with male participants. In contrast, males demonstrated relatively higher mean levels of fasting blood glucose and HbA1c (%). The observed differences suggest a less favourable lipid profile among female participants, while indicators of long-term glycaemic control were comparatively higher in males. Apart from AST and urea, liver enzymes, renal markers, electrolytes, and mineral parameters did not show statistically significant sex-based differences (p > 0.05), indicating overall biochemical comparability between male and female participants within the study population.
Table 2: Comparison of biochemical parameters between male and female
|
Parameter |
Total |
Male |
Female |
P-value |
|
(Mean ± SD) |
(Mean ± SD) |
(Mean ± SD) |
||
|
FBS (mmol/L) |
8.622 ± 2.77 |
8.795 ± 2.82 |
8.23 ± 2.59 |
0.192 |
|
2 hur ABF (mmol/L) |
9.94 ± 3.54 |
9.89 ± 3.41 |
9.99 ± 3.98 |
0.863 |
|
HbA 1c (%) |
8.468 ± 2.06 |
8.69 ± 2.14 |
7.96 ± 1.79 |
0.0203* |
|
Creatinine (mg/dl) |
1.276 ± 0.75 |
1.22 ± 0.62 |
1.41 ± 0.97 |
0.1755 |
|
Urea (mg/dl) |
49.74 ± 44.36 |
42.89 ± 28.99 |
65.45 ± 64.79 |
0.017* |
|
Bilirubin (mg/dL) |
0.8857± 0.54 |
0.85 ±0.40 |
0.96 ± 0.78 |
0.325 |
|
ALT (U/L) |
43.15 ± 38.56 |
41.16 ± 32.78 |
47.69 ± 49.00 |
0.372 |
|
AST (U/L) |
35.1 ± 34.28 |
30.91 ± 26.44 |
44.69 ± 46.19 |
0.044* |
|
ALP(U/L) |
106 ± 64.43 |
100.67 ± 54.93 |
118.07 ± 80.86 |
0.153 |
|
Cholesterol(mg/dL) |
171.6 ± 52.95 |
164.36 ± 48.94 |
188.11 ± 57.85 |
0.001* |
|
HDL (mg/dL) |
32.99 ± 7.03 |
32.90 ± 7.09 |
33.19 ± 6.91 |
0.802 |
|
LDL (mg/dL) |
104.9 ± 38.57 |
100.37 ± 37.98 |
115.28 ± 37.92 |
0.0176* |
|
Triglycerides |
195.7 ± 126.98 |
194.11 ± 128.39 |
199.51 ± 123.60 |
0.7913 |
|
Sodium (mmol/L) |
136.2 ± 7.68 |
135.93 ± 7.13 |
136.87 ± 8.77 |
0.4873 |
|
Potassium (mmol/L) |
4.03 ± 0.79 |
4.03 ± 0.73 |
4.03 ± 0.90 |
0.991 |
|
Chloride (mmol/L |
102.9 ± 8.97 |
102 ± 6.73 |
104.91 ± 12.45 |
0.11 |
|
Albumin (gm/L) |
33.58 ± 5.83 |
33.68 ± 5.13 |
33.35 ± 7.17 |
0.756 |
|
Magnesium (mmol/L) |
0.7915 ± 0.19 |
0.78 ± 0.19 |
0.81 ± 0.19 |
0.384 |
|
Calcium (mmol/L) |
2.18 ± 0.20 |
2.18 ± 0.19 |
2.19 ± 0.21 |
0.706 |
*P-values were calculated using independent t-test. A p-value < 0.05 was considered statistically significant
Serum Tumor Marker
Serum tumor marker levels are summarized in Table 3 and are expressed as mean ± standard deviation along with minimum and maximum values. Among the assessed markers, AFP, CA-125, and CA 15-3 exhibited wide variability, reflected by large standard deviations and broad ranges, indicating substantial inter-individual variation within the study population. CEA and PSA demonstrated comparatively lower mean values and narrower distributions, suggesting relative stability across participants. The wide dispersion observed for AFP, CA-125, and CA 15-3 suggests that tumor marker concentrations may be influenced by non-malignant factors, including metabolic and biochemical alterations, rather than reflecting underlying malignancy in all cases. Overall, the distribution pattern highlights the heterogeneity of serum tumor marker levels in adult patients and supports cautious interpretation of isolated tumor marker elevations, particularly in populations with metabolic dysregulation.
Table 3: Distribution of serum tumor marker levels in the study population
|
Tumor marker |
Mean ± Sd |
Maximum |
Minimum |
|
CEA (ng/ml) |
2.645 ± 4.49 |
52 |
0.12 |
|
AFP (ng/ml) |
17.08 ± 119.21 |
1600 |
0 |
|
CA_19.9 (U/ml) |
25.51 ± 64.58 |
666.3 |
0 |
|
CA_125 (U/ml) |
32.26 ± 91.61 |
1200 |
0 |
|
CA_15.3 (U/ml) |
11.9 ± 108.36 |
583 |
0 |
|
PSA (ng/ml) |
1.73 ±3.55 |
33 |
0 |
Abnormal Biochemical and Tumor Marker
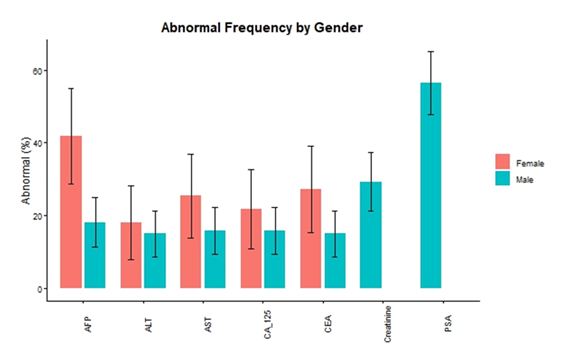
The frequency of abnormal biochemical and tumor marker values stratified by gender is presented in Table 4 and illustrated in Figure 1. Abnormality was defined based on established reference ranges. Among liver enzymes, abnormality was more frequent in females compared to males. Renal function abnormality, assessed by creatinine levels, was observed in 29.37% of male participants, whereas no abnormal creatinine values were detected among females. Regarding tumor markers, CEA abnormality was more prevalent in females than males. A pronounced gender-based difference was also observed for AFP, with abnormal values detected in 41.82% of females compared to 18.25 % of males. CA-125 abnormality was more common in females than males while PSA abnormality was observed in 56% of male participants, as expected due to gender-specific expression. No abnormal values were observed for CA 19-9 and CA 15-3 in either gender.
Table 4: Gender-wise abnormal biochemical and tumor markers (%)
|
Variables |
Abnormality % |
|
|
Male |
Female |
|
|
ALT |
15.08 |
18.18 |
|
AST |
15.87 |
25.45 |
|
Creatinine |
29.37 |
NA |
|
CEA |
15.08 |
27.27 |
|
AFP |
18.25 |
41.82 |
|
CA_19_9 |
NA |
NA |
|
CA_125 |
15.87 |
21.82 |
|
CA_15_3 |
NA |
NA |
|
PSA |
56.35 |
NA |
Correlation Analysis
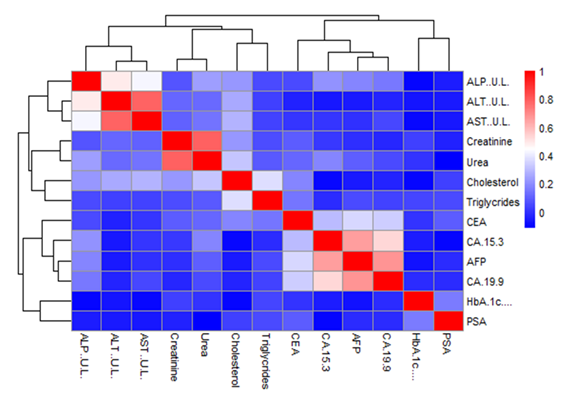
The hierarchical cluster correlation heatmap showing the relationships among biochemical parameters, glycemic index, and serum tumor markers shown in Figure 2. Strong positive correlations were observed among liver enzymes (ALT, AST, and ALP), indicating shared hepatic functional pathways. Renal function markers, urea and creatinine, also clustered together, demonstrating a moderate positive correlation. Lipid parameters, particularly total cholesterol and triglycerides, formed a separate cluster, suggesting metabolic interrelatedness. HbA1c (%) showed modest positive correlations with selected biochemical parameters and tumor markers, reflecting the influence of long-term glycemic control on metabolic and biochemical status. Among tumor markers, AFP, CA 19-9, and CA 15-3 exhibited moderate to strong positive inter-correlations, forming a distinct cluster. This suggests common regulatory influences, possibly related to metabolic stress or systemic inflammation rather than malignancy alone. CEA showed weaker correlations with other tumor markers and biochemical parameters, indicating a more independent behavior. PSA displayed minimal correlation with most biochemical parameters and tumor markers, supporting its relative specificity and gender-related expression. Overall, the clustering pattern highlights distinct biochemical domains-hepatic, renal, metabolic, and tumor marker clusters—while also demonstrating cross-domain interactions influenced by glycemic status.
Figure 2: Hierarchical cluster correlation heatmap showing relationships among biochemical parameters, HbA1c (%), and serum tumor markers. Color intensity represents correlation strength (red = strong positive correlation; blue = weak or negative correlation). Dendrograms indicate clustering based on similarity patterns
Multivariable Regression Analysis of Tumor Markers
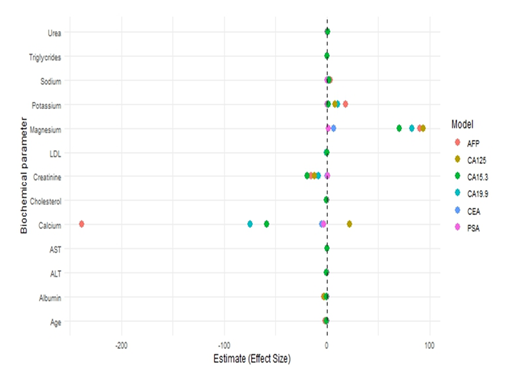
Multivariable linear regression analyses were performed to evaluate independent associations between demographic and clinico-biochemical parameters and serum tumor marker levels (CEA, AFP, CA19-9, CA-125, PSA, and CA15-3). The multi variable regression analysis presented in Table 5 and illustrated in Figure 3. Age showed a significant positive association with CEA levels (β = 0.044, p = 0.040). Among biochemical parameters, serum magnesium was strongly and positively associated with CEA (β = 6.93, p < 0.001), whereas serum calcium demonstrated a significant inverse association (β = −4.67, p = 0.012). Liver enzymes, lipid profile, renal function markers, electrolytes, and triglycerides did not show significant independent associations with CEA. Alpha-Fetoprotein (AFP) levels were negatively associated with age (β = −1.72, p = 0.002). Significant positive associations were observed with serum sodium (β = 3.33, p = 0.005), urea (β = 0.81, p = 0.007), and magnesium (β = 90.72, p = 0.036). In contrast, serum calcium exhibited a strong inverse relationship with AFP (β = −238.47, p < 0.001). Other parameters, including liver enzymes, lipid markers, creatinine, and triglycerides, were not independently associated. ALT showed a significant negative association with CA19-9 levels (β = −0.39, p = 0.040). Additionally, albumin was inversely associated with CA19-9 (β = −2.58, p = 0.002). Serum magnesium demonstrated a positive association (β = 82.72, p < 0.001), while serum calcium was inversely related to CA19-9 levels (β = −74.77, p = 0.005). No significant associations were observed with age, renal markers, lipid profile, or triglycerides. CA-125 levels were negatively associated with serum albumin (β = −2.56, p = 0.039) and positively associated with serum magnesium (β = 93.69, p = 0.011). Other variables, including age, liver enzymes, renal parameters, lipid profile, electrolytes, calcium, and triglycerides, did not demonstrate significant independent associations. Prostate-Specific Antigen (PSA) showed a strong positive association with age (β = 0.055, p < 0.001). Urea levels were inversely associated with PSA (β = −0.023, p = 0.006), while serum calcium also demonstrated a significant negative association (β = −3.10, p = 0.019). No significant relationships were observed with liver enzymes, lipid parameters, creatinine, albumin, magnesium, or triglycerides. CA15-3 levels were positively associated with serum sodium (β = 2.25, p < 0.001), urea (β = 0.65, p < 0.001), and magnesium (β = 70.27, p = 0.001). In contrast, creatinine (β = −19.26, p = 0.027) and serum calcium (β = −58.44, p = 0.013) showed significant inverse associations. ALT and cholesterol showed borderline associations, while age, AST, LDL, albumin, potassium, and triglycerides were not independently associated. Across all tumor markers, serum magnesium consistently demonstrated positive independent associations, while serum calcium showed predominantly inverse associations. Age remained an important determinant for CEA, AFP, and PSA. Renal markers (urea and creatinine) and electrolytes (particularly sodium) displayed marker-specific associations, whereas liver enzymes and lipid parameters showed limited independent influence after multivariable adjustment.
Table 5: Multi factor Regression analysis for tumor marker
|
Tumor marker |
Biochemical parameter |
β Estimate |
St. Error |
t value |
p- value |
|
CEA |
Age |
0.044 |
0.021 |
2.068 |
0.0402 |
|
CEA |
Magnesium |
6.925 |
1.694 |
4.088 |
6.74×10-5 |
|
CEA |
Calcium |
4.667 |
1.841 |
2.535 |
0.0122 |
|
AFP |
Sodium |
3.327 |
1.163 |
2.86 |
0.0048 |
|
AFP |
Urea |
0.811 |
0.298 |
2.721 |
0.0072 |
|
AFP |
Magnesium |
90.72 |
42.83 |
2.118 |
0.0357 |
|
AFP |
Age |
1.717 |
0.538 |
3.194 |
0.00167 |
|
AFP |
Calcium |
238.47 |
46.56 |
5.122 |
8.27×10-07 |
|
CA19.9 |
ALT |
0.3923 |
0.19 |
2.069 |
0.0401 |
|
CA19.9 |
Albumin |
-2.576 |
0.817 |
3.152 |
0.0019 |
|
CA19.9 |
Magnesium |
82.72 |
24.36 |
3.395 |
0.0009 |
|
CA19.9 |
Calcium |
74.77 |
26.43 |
2.829 |
0.0052 |
|
CA125 |
Albumin |
2.56 |
1.24 |
2.076 |
0.0394 |
|
CA125 |
Magnesium |
93.69 |
36.65 |
2.557 |
0.0115 |
|
PSA |
Age |
0.055 |
0.015 |
3.645 |
0.0004 |
|
PSA |
Urea |
0.023 |
0.008 |
2.76 |
0.0064 |
|
PSA |
Calcium |
3.1 |
1.313 |
2.361 |
0.0194 |
|
CA15.3 |
Sodium |
2.25 |
0.582 |
3.873 |
0.0002 |
|
CA15.3 |
Creatinine |
19.26 |
8.65 |
2.226 |
0.0274 |
|
CA15.3 |
Urea |
0.647 |
0.15 |
4.343 |
2.43×10-05 |
|
CA15.3 |
Magnesium |
70.27 |
21.43 |
3.279 |
0.0013 |
|
CA15.3 |
Calcium |
58.44 |
23.3 |
2.509 |
0.0131 |
This figure 3 illustrates adjusted β coefficients derived from multivariable linear regression models evaluating associations between clinico-biochemical parameters and serum tumor markers (AFP, CA-125, CA15-3, CA19-9, CEA, and PSA). Points represent effect estimates for each biochemical variable within individual tumor marker models. Positive coefficients indicate increased tumor marker concentrations with higher parameter values, whereas negative coefficients indicate inverse associations. Overall, most biochemical parameters exhibited modest effect sizes clustered around zero, suggesting weak or non-significant independent associations after multivariable adjustment. In contrast, selected parameters demonstrated pronounced marker-specific effects. Serum magnesium showed a strong positive association across multiple tumor markers, particularly CA15-3, CA19-9, and CEA, indicating that higher magnesium levels were independently associated with elevated tumor marker concentrations. Conversely, serum calcium exhibited strong negative associations with AFP and CA19-9, suggesting higher tumor marker levels at lower calcium concentrations. Renal function markers displayed differential effects, with creatinine showing inverse associations and urea demonstrating positive associations with selected tumor markers, highlighting the potential role of renal metabolism and clearance in tumor marker variability. Electrolytes, including sodium and potassium, showed moderate positive associations most notably with CA19-9 and CA15-3-whereas lipid parameters (LDL and triglycerides) demonstrated minimal or near-null effects. Liver enzymes (ALT and AST), age, and albumin showed relatively small effect estimates, indicating limited independent contribution to tumor marker variability in the adjusted models.
Discussion
This cross-sectional study evaluated the association between glycemic status with clinico-biochemical parameters and serum tumor marker profiles in adult patients, with particular emphasis on sex-specific differences, biochemical abnormalities, correlation patterns, and multivariable-adjusted relationships. The findings highlight that glycemic dysregulation is accompanied by distinct alterations in metabolic, mineral, renal, and tumor marker profiles, even in the absence of overt malignancy.
Sex-specific differences in biochemical parameters
Among the evaluated biochemical variables, HbA1c%, urea, AST, total cholesterol, and LDL cholesterol demonstrated statistically significant sex-based differences, with higher mean values observed predominantly in female participants. These findings suggest that female patients may experience a greater metabolic burden associated with glycemic dysregulation, particularly in lipid metabolism and hepatic function. The higher HbA1c% values observed among females may reflect sex-related differences in insulin sensitivity, body fat distribution, hormonal influences, or health-seeking behaviors. Elevated urea and AST levels further suggest potential sex-dependent variations in renal handling and hepatic stress in the context of altered glycemic control.
Tumor marker profiles and abnormality frequencies
Although mean tumor marker concentrations largely remained within reference ranges, a substantial proportion of participants exhibited abnormal tumor marker levels, with notable sex differences. Females showed higher abnormality frequencies for CEA, AFP, AST, ALT, and CA-125, whereas PSA abnormalities were exclusively observed among males, as expected. Importantly, AFP and CEA demonstrated comparatively higher abnormality rates in females, indicating that glycemic and metabolic dysregulation may influence tumor marker expression differently by gender. These findings support the growing evidence that tumor markers may be modulated by non-malignant metabolic conditions, potentially reducing their specificity in populations with metabolic disorders12.
Correlation patterns and clustering behavior
Correlation analysis and hierarchical clustering revealed distinct groupings between biochemical parameters and tumor markers. Liver enzymes, lipid parameters, and renal markers demonstrated inter-correlations consistent with metabolic syndrome-related clustering, while tumor markers formed separate but partially overlapping clusters13. Notably, magnesium and calcium displayed strong correlations with multiple tumor markers, suggesting a potential modulatory role of mineral homeostasis in tumor marker variability. HbA1c% also demonstrated clustering proximity to selected tumor markers, reinforcing the relationship between glycemic status and tumor marker profiles14.
Multivariable regression findings
Multivariable regression analyses identified magnesium and calcium as the most consistent independent predictors across multiple tumor marker models. Serum magnesium showed strong positive associations with CEA, AFP, CA19-9, CA15-3, and CA-125, while calcium demonstrated inverse associations with AFP, CA19-9, CEA, PSA, and CA15-3. These findings suggest that mineral imbalance may significantly influence tumor marker concentrations independent of traditional biochemical confounders. Renal markers exhibited marker-specific effects15. Urea showed positive associations with AFP, PSA, and CA15-3, whereas creatinine demonstrated inverse associations, indicating that renal clearance and protein metabolism may contribute to circulating tumor marker levels16. Age remained an independent predictor for CEA and PSA, consistent with established age-related increases in these markers. In contrast, lipid parameters (LDL, triglycerides) and liver enzymes (ALT, AST) demonstrated minimal independent associations after multivariable adjustment, suggesting that their observed univariate relationships with tumor markers may be mediated through other metabolic or renal pathways. Albumin showed limited inverse associations, reflecting its role as a marker of nutritional and inflammatory status rather than a direct determinant of tumor marker variability17.
Clinical implications
The findings underscore that tumor marker interpretation in patients with altered glycemic status should be approached with caution, as metabolic, renal, and mineral disturbances may influence tumor marker concentrations in the absence of malignancy. In particular, magnesium and calcium levels appear to be important confounders and should be considered when evaluating mildly elevated tumor markers in metabolic populations. These results also highlight the importance of gender-specific evaluation, as females demonstrated a higher frequency of biochemical and tumor marker abnormalities.
Conclusion
This study demonstrates that glycemic status is associated with significant alterations in clinico-biochemical parameters and serum tumor marker profiles in adult patients. Gender-specific differences were evident, with females exhibiting higher mean values and abnormality frequencies for several metabolic and tumor markers. Multivariable analyses identified serum magnesium and calcium as key independent determinants of tumor marker variability, while renal function markers further influenced marker concentrations. These findings emphasize the need for careful interpretation of tumor markers in individuals with metabolic dysregulation and support the integration of metabolic and mineral assessments in clinical evaluation. Future longitudinal studies are warranted to clarify the clinical relevance of these associations and their implications for cancer risk assessment.
Study Limitations
This study is limited by its cross-sectional design, which precludes causal inference. The absence of longitudinal follow-up and cancer outcome data limits the ability to determine the prognostic significance of tumor marker elevations. Additionally, potential confounders such as medication use, dietary intake, and inflammatory markers were not included. Despite these limitations, the comprehensive multivariable approach strengthens the robustness of the findings.
Conflict of Interest: The authors declare no conflict of interest.
References
- Taguchi M, Bouchi R, Fukuda T, et al. Clinical significance of tumor markers in patients with type 2 diabetes: a retrospective observational study. Diabetology international 14 (2023): 40-50.
- Turati F, Galeone C, Augustin LS, et al. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients 11 (2019): 2342.
- Sieri S, Krogh V. Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutrition, Metabolism and Cardiovascular Diseases 27 (2017): 18-31.
- Singla R, Goyal A, Mahajan B, et al. Effect of glycemic status on serum CEA and CA 19-9 levels in patients of diabetes mellitus in northern India. Int J Health Sci Res 8 (2018): 67-72.
- Samiul M, Mohi MK, Akter F, et al. Association between hepatocellular carcinoma and diabetes mellitus. Journal of Primeasia 6 (2025): 1-7.
- Shang X, Song C, Du X, et al. The serum levels of tumor marker CA19-9, CEA, CA72-4, and NSE in type 2 diabetes without malignancy and the relations to the metabolic control. Saudi medical journal 38 (2017) :204.
- Chen PC, Lin HD. Reversible high blood CEA and CA19-9 concentrations in a diabetic patient. Libyan Journal of Medicine 7 (2012).
- Ure O, Ata N, Kucukazman M, et al. Glycemic control influences CA19-9, CEA and ferritin levels in type 2 diabetes. InEndocrine Abstracts 29 (2012).
- Meng M, Shi LL. Serum tumor markers expression (CA199, CA242, and CEA) and its clinical implications in type 2 diabetes mellitus. World Journal of Diabetes 15 (2024): 232.
- Bashir MS, Sayem MA, Das SS, et al. High Viral Load is a Risk Factor for Hepatocellular Carcinoma: Clinical and Laboratory Insights from a Cross-Sectional Study. Integrative Biomedical Research 9 (2025): 1-8.
- Chandran V, Rao GM, Rao D. Correlation of CA 19-9 with HbA1c in prediabetic and diabetic groups. Biomedicine 41 (2021): 737-41.
- Lee EJ, Seo HA, Kim EH. The Difference of CA 19-9 Level among diabetes, prediabetes and healthy control. InInternational Congress of Diabetes and Metabolism (2015): 157-157.
- Keum N, Yuan C, Nishihara R, et al. Dietary glycemic and insulin scores and colorectal cancer survival by tumor molecular biomarkers. International journal of cancer 140 (2017): 2648-56.
- Lawal AO, Adeleye JO, Esan A, et al. Frequency and Correlates of Elevated Serum Carbohydrate Antigen 19-9 Levels in a Cohort of Nigerians with Type 2 Diabetes Mellitus. Journal of Diabetology 16 (2025): 77-82.
- Ata N, Dal K, Kucukazman M, et al. The effect of glycemic control on CEA, CA 19-9, amylase and lipase levels. Open Medicine 17 (2014): 8.
- Reddy KS, Pandiaraj IP, Gaur A, et al. Serum tumor markers: Can they clinically implicate in type 2 diabetes mellitus?. World Journal of Diabetes 15 (2024): 1648.
- Zhuang Y, Cai Q, Hu X, et al. Elevated serum CA199 levels in patients suffering type 2 diabetes vs. various types of cancer. BMC Endocrine Disorders 24 (2024): 9.