Chemical Inhibition and Ecological Impact of Prosopis juliflora in Saudi Arabia
Alfagham, Alanoud1*, Al-atawi, Reem2, Alothmani, Yasmeen3, Alsadoun, Aseel1
1Department of Botany and Microbiology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
2Department of Zoology, College of Science, King Saud University, Riyadh 1145, Saudi Arabia
3The Royal Commission for AlUla (RCU), Al Safarat, Riyadh 12512, Saudi Arabia
*Corresponding Authors: Alanoud Tala Alfagham, Department of Botany and Microbiology, College of Science, King Saud University, Riyadh 11451, Saudi Arabia.
Received: 27 July 2025; Accepted: 12 August 2025; Published: 21 August 2025
Article Information
Citation: Alfagham, Alanoud, Al-atawi, Reem, Alothmani, Yasmeen, Alsadoun, Aseel. Chemical Inhibition and Ecological Impact of Prosopis juliflora in Saudi Arabia. International Journal of Plant, Animal and Environmental Sciences. 15 (2025): 132-139.
Share at FacebookAbstract
Prosopis juliflora, an invasive species known for its allelopathic properties, poses a significant threat to biodiversity, particularly in arid regions like the Kingdom of Saudi Arabia (KSA). This investigation sheds light on the chemical mechanisms underlying its allelopathic effects using Fourier- Transform Infrared (FTIR) spectroscopy and assesses the influence of leaf extract and soil solution on the germination of native plant species (Acacia gerrardii, Horwoodia dicksoniae and Panicum turgidum). The study identifies critical allelochemicals responsible for suppressing native plant species under study. These include nitriles, alkynes, thiocyanates, carbonyl groups, and alcohols or amines in plant extracts and soil solutions, although plant extracts had the highest impact on seed germination. The results suggest these compounds interfere with plant proteins and cellular processes, reducing growth and biodiversity loss. The paper concludes with a call for urgent biodiversity conservation measures to mitigate the adverse impact of P. juliflora.
Keywords
Allelopathy; Prosopis juliflora; Chemical inhibition; Plant extract; FTIR spectroscopy; Ecological impact; Biodiversity conservation
Allelopathy articles; Prosopis juliflora articles; Chemical inhibition articles; Plant extract articles; FTIR spectroscopy articles; Ecological impact articles; Biodiversity conservation articles.
Article Details
1. Introduction
Invasive plants are non-native plants that, upon introduction, spread rapidly and cause significant damage to ecosystems, biodiversity, human health, and economies. These plants disrupt the ecological equilibrium by competing with native plants, resulting in changes in community structures and ecosystem functions [1]. Significant economic and health challenges in several ecosystems are associated with invasive plant species [2]. Management of invasive plants entails substantial expenditures, necessitating substantial financial resources for management and mitigation efforts [3]. Furthermore, invasive plants can adversely affect human health by causing allergies and skin irritation [4]. Effective management strategies are needed to mitigate these impacts, which disrupt agricultural productivity and local economies. The 1992 Convention on Biological Diversity (CBD) state these strategies for management: Preventing the spread of invading species is the most crucial approach, aiming to deter their emergence. When prevention fails, early detection, rapid response, and potential eradication are essential to control new invasions. Physical removal, chemical treatments, and biological control are some of the long-term management strategies. Sustainable control of invasive plants requires integrated management strategies that combine these methods with a focus on prevention. Long-term management costs and prevent irreversible ecological changes [2,4]. Prosopis juliflora, commonly known as mesquite, has recently been a major invasive plant in Saudi Arabia, it belongs to the family Fabaceae, order Fables. It is characterised by its deep root system, compound leaves, and thorny branches, the plant can grow up to 8 meters tall and produce numerous seeds [5,6]. Moreover, it is a fast-growing evergreen tree or spiny shrub native to South and Central America and was introduced globally from the late 1970s to early 1980s in order to address deforestation and desertification issues. However, its ability to grow quickly, produce large quantities of seeds, and thrive in diverse climatic and soil conditions has made it one of the 100 most invasive alien plant species worldwide [7]. The seeds are dispersed by livestock, wild animals, and water, allowing the plant to colonise new areas rapidly. Additionally, P. juliflora can tolerate a wide range of soil types, including saline and alkaline soils, and can survive in extremely arid conditions [8,9]. The impact of P. juliflora is not only due to its ability to be highly spared but also to its allelopathy effect [10]. In 1937, Hans Molisch introduced the term "allelopathy" to describe all direct and indirect effects resulting from biochemical substances transferred from one plant to another. The term "allelopathy" is derived from the Greek words "allelon" (mutual) and "pathos" (harm or suffering), highlighting the mutual influence and potential negative consequences of these biochemical interactions, the International Allelopathy Society (IAS) expanded the definition in 1996 to encompass any process involving secondary metabolites produced by plants, microorganisms, viruses, and fungi that influence the growth and development of agricultural and biological systems [11].
Plant allelopathy involves the interaction of plant receptors and plant donors. The chemical compounds released by one plant affect the growth and development of the other; these interactions can influence various aspects of agricultural and natural ecosystems [11]. Prosopis juliflora contains a variety of chemical compounds that contribute to its allelopathic effects on neighbouring plants. These compounds include: Phenols: Such as caffeic acid and ferulic acid, which disrupt the cell membranes of neighbouring plants and affect ion absorption and enzymatic activities [12]. Alkaloids, Including juliflorine, julifloricine, and juliprosopine, are known for their growth-inhibiting effects on other plants by interfering with cell division and photosynthesis [13]. Flavonoids: Compounds like quercetin and kaempferol, which inhibit plant growth by affecting metabolic processes within plant cells, free Sugars: Such as glucose and fructose, which may influence plant growth by altering the water balance within plant cells [14,15]. Volatile Compounds: Such as terpenes, which can have inhibitory effects on neighbouring plants through evaporation into the surrounding environment and interaction with other plants [16]. These chemical compounds affect native plants that grow near them through several mechanisms. One of these mechanisms is the disruption of cell membranes by increasing phenols and the permeability of cell membranes, causing the loss of essential ions and nutrients and leading to cell death [12]. Also, Phenolic and flavonoid compounds can cause an alteration of ion balance by affecting plant ion absorption, leading to an imbalance necessary for average growth [9]. Moreover, Alkaloids affect the activity of enzymes necessary for photosynthesis and respiration, hindering the growth of neighbouring plants [13]. Some compounds, like juliprosopine, prevent cell division, limiting the formation of new plant tissues and hindering growth [17].
Several studies have investigated the allelopathic effects of P. juliflora in different regions, providing valuable insights into its impact on native plant species and ecosystems. For instance, a study conducted in the Middle Awash region of Ethiopia found that P. juliflora invasion significantly reduced the germination and growth of native species such as Acacia nilotica and Cenchrus cillaris [6]. The study highlighted that the allelopathic effects were concentration-dependent, with higher concentrations of P. juliflora extracts causing more significant growth inhibition. In another study, researchers in Ethiopia examined the impact of P. juliflora on tropical crops under laboratory conditions. They found that all parts of the plant, including leaves, roots, and litter, contained water-soluble allelochemicals capable of inhibiting the germination and growth of crops like maize and cotton [8]. These findings underscore the potential threat of P. juliflora to both agricultural productivity and native plant communities.
This research aims to investigate the allelopathic effects of aquacise leaf extract and soil solution of Prosopis juliflora, focusing on its impact on native plant species seed germination and evaluating its impact on biodiversity.
2. Experimental
2.1 Site selection and sample collection
Soil and plant leaves from under the canopy of Prosopis juliflora were collected from the southwest of Riyadh, Saudi Arabia. Native plants (Acacia gerrardii, Horwoodia dicksoniae and Panicum turgidum) were selected from Seed banks.
2.2 Preparation of leaf extraction
The plant samples were dried in the shade at room temperature and then ground into a fine powder. Ten grams of the fine powder were soaked in 100 ml of distilled water for 24 hours. The solution was filtered to obtain a concentrated extract (100%).
2.3 Preparation of soil solution
Measure out 20 gm of soil that has been dried in the air and is smaller than 2 ml in size. Place the soil in a bottle and add 100 ml of deionised water. 15-rpm mechanically shake for 1 hour.
2.4 Seed germination measurement.
Seeds of plants under study (Acacia gerrardii, Horwoodia dicksoniae and Panicum turgidum) were collected from the seed bank in Riyadh, Saudi Arabia. All damaged and empty seeds were discarded using a flotation process in distilled water prior to germination tests. The surface of the seeds was sterilised with chlorine water (5ml chlorine 95ml water) for 5-10 minutes, then rinsed several times with distilled water until it was confirmed that there was no chlorine smell. Ten seeds at three replicates for each treatment were sowed in Petri dishes on a single layer of filter paper saturated with distilled water for the germination test. Seeds germinating 7-10 days after the start of the experiment were monitored. The seeds were considered to have germinated when their radicle or germ length reached approximately 1 mm. The germinability of the seeds was observed on the tenth day. Each time, seed germination was calculated as the flowing equation according to (Al-Imam et al. 2016).
Germination %= (G. Seed / T. Seed)* 100
The G. Seed is the number of germinated seeds, and the T. Seed is the total number of seeds.
Ten seedlings per dish were randomly selected after germination measurement, and the height of the root seedlings (S) was measured with a standard ruler (cm). The seed vitality index (VI) was then calculated as follows: VI = GI∗S, where GI is the germination index, and S is the average height of the root seedlings.
2.5 Chemical Analysis of Extracts
One mL of each extract was taken for FTIR analysis (Fourier transform infrared spectroscopy). FTIR was used to analyse the spectra and identify the chemical compounds in the plant extract and soil solution [18-20].
3. Results and Discussion
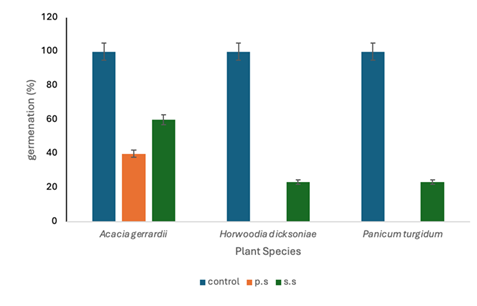
The presence of invasive plants in any ecosystem can impact on the structure and composition of plant communities directly through its root exudates, consequently influence on the emergence and early growth of other native seedlings [21]. In this study, the findings on the allelopathic effects of P. juliflora was significantly observed. They provide crucial insights into how this invasive species disrupt native plant communities. These insights can help predict the long-term ecological impacts on biodiversity and ecosystem stability. The results showed that plant extract had a significant inhibition (p > 0.014) on seed germination of plants under study with the parallel of root vitality compared with control plants, and soil solution had less effect on seed germination compared with plant extract to all plants under study.
The results showed that Acacia gerrardii seed germination was affected by plant extract (40%), Horwoodia dicksoniae (0%) and Panicum turgidum (0%) (Figure 1, Table 1). Although soil solution had decreased the germination ratio, Acacia gerrardii seed germination was 60%, Horwoodia dicksoniae and Panicum turgidum (23%) (Figure 1, Table 1). From the above results, plant extract has a higher effect on seed germination than soil solution. However, the chemical components remained in both treatments and had the same effect on plant germination. The negative impact of plant extracts on seed germination and root vitality mainly because of allelopathic chemical compounds produced as root exudates from the invasive plant [22] which alter the soil chemical composition [5,23], hence the growth of subsequent natives will be limited. These results align with several studies which have shown that aqueous extracts from the leaves, roots, and litter of Prosopis juliflora negatively affect the germination and growth of various native plant species [8,12]. Another study conducted field surveys and laboratory experiments to assess the allelopathic effects of P. juliflora on native plants. The aqueous extracts of P. juliflora were found to significantly reduce the germination and seedling growth of several native species such as Acacia tortilis, Prosopis cineraria, Sueda aegyptica, and Halopeplis perfoliata. This indicates that P. juliflora can effectively outcompete native plants by releasing allelopathic chemicals that inhibit their growth [5]. These findings not only confirm the need for further research in this area but also open up new avenues for understanding the complex interactions between invasive and native plant species. The implications of this study underscore the urgent need for further research and conservation efforts in this area.
|
Plant species |
Germination |
Root vitality |
||||
|
control |
p.s |
s.s |
control |
p.s |
s.s |
|
|
Acacia gerrardii |
100 ±0 |
40 ± 0.0.57 |
60 ± 0 |
6.5 |
1 |
3.16 |
|
Horwoodia dicksoniae |
100 ±0 |
0 |
23.3 ±0.33 |
1.83 |
0 |
0.67 |
|
Panicum turgidum |
100 ±0 |
0 |
23.3 ±00.66 |
2 |
0 |
1.17 |
|
F |
5.115 |
|||||
|
Sig. |
0.014 |
|||||
Table 1: The effect of plant extract (p.s) and soil solution (s.s) on seed germination of the plants under study is compared with that of the control—and Root vitality.
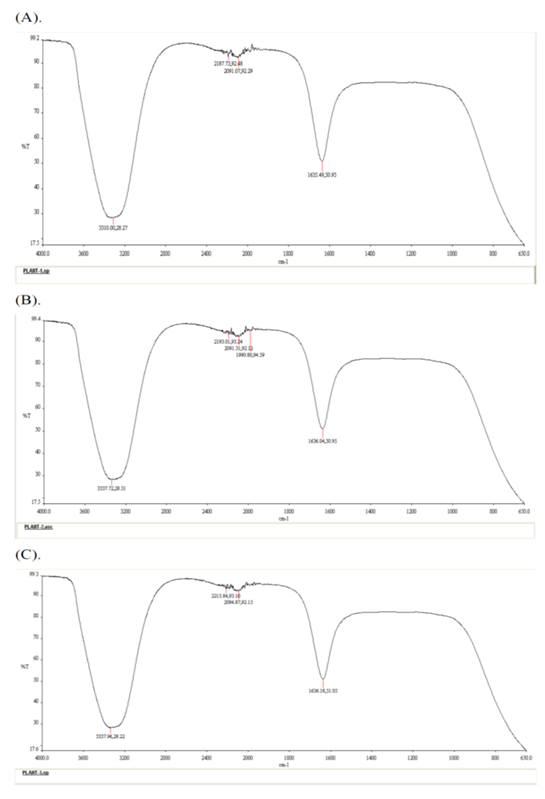
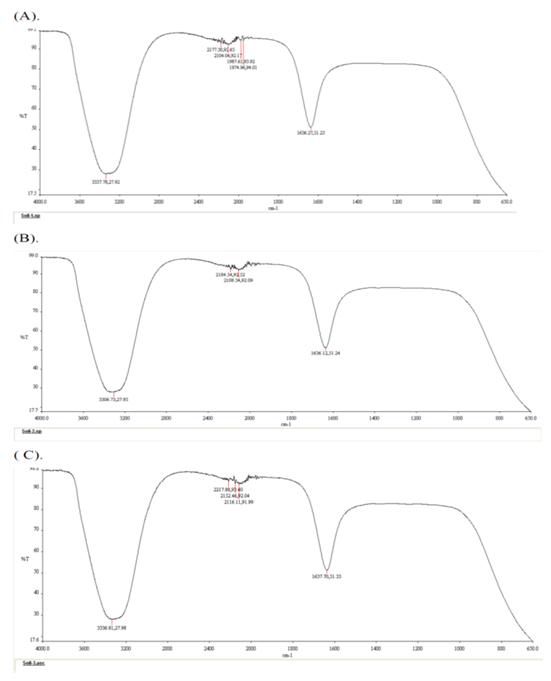
Allelopathy involves the chemical inhibition of one plant species by releasing toxic substances. The FTIR spectroscopy experiments have identified different chemical contents in the plant extract and soil solution under the canopy of Prosopis juliflora, as shown in Figure 2 to Figure 3. The absorption spectra provided the wave numbers (cm –1) of the prominent peaks listed in Table 2. Compared with the previous study, it found that functional group analysis indicates the presence of nitriles, alkynes or thiocyanates, carbonyl groups, and alcohols or amines in Plant extract, which are mainly attributed to phenols, ketene and isothiocyanate (Figure 2, Table 2) and in soil solutions, phenols, ketene, allene and azide had a high concentration (Figure 3, Table 2). Our findings came to support the previous reports regarding the allelopathic impacts of such compounds on plant metabolism [24,25]. These results suggest that alteration in the soil nutrient content by invasive species is vital to facilitate invasion success [26] in a way to compete with native species [27]. Studies indicate these compounds can disrupt ionic transport across cell membranes, impairing essential cellular functions [5]. Alkynes and thiocyanates are known for their toxic effects on plant cells [28,29]. Such allelochemicals may alter the cell membranes of plant cell which increase cell wall permeability causing ionic imbalance and growth inhibition [30,31]. These findings came in agreement with previous researches [32,33] who confirmed that the invasive plants influence carbon nitrogen-cycles. Carbonyl groups, such as aldehydes and ketones, can react with amino acids in proteins, forming new chemical bonds that alter protein structure and function. This alteration can inhibit vital enzymes and cellular functions [5]. Alcohols and amines can affect the fluidity and stability of cell membranes, disrupting membrane functions such as ion transport and cell protection [34,35].
These compounds may interfere with essential biological processes, including water balance. The invasion of P. juliflora reduces species richness and alters plant community structures. Its dense canopy and allelopathic effects created an unfavourable environment for native flora, resulting in decreased biodiversity and disrupted ecosystem services.
In KSA, where biodiversity is threatened due to harsh environmental conditions, the spread of P. juliflora poses a significant risk to native plant species and overall ecosystem health [36]. This spread underscores the urgent need for immediate action to curb this invasive species' spread and restore our ecosystems' balance [37-40].
Figure 2: FT-IR spectra of Plant Extract: (A). Plant Sample 1, (B). Plant sample 2, (C). plant sample 3, Main Wavelengths: 2187.73 cm<sup>-1</sup>, 2081.07 cm<sup>-1</sup>, 1635.49 cm<sup>-1</sup>, 3310.00 cm<sup>-1</sup>. Functional Group Analysis: Indicates the presence of nitriles, alkynes or thiocyanates, carbonyl groups, and alcohols or amines. (chem.libretexts).
Figure 3: FT-IR spectra of soil solution: (A) Soil solution Sample 1, (B) Soil solution Sample 2, (C) Soil solution Sample 3, Main Wavelengths: 1974.96 cm<sup>-1</sup>, 1636.27 cm<sup>-1</sup>, 3337.78 cm<sup>-1</sup>. Functional Group Analysis: Indicates the presence of alkynes or thiocyanates, carbonyl groups, and alcohols or amines. (chem.libretexts).
|
S. No. |
Peak (cm–1) |
Assignment |
|
Plant extract sample 1 |
3310 |
O-H present in phenols. |
|
2187 |
C=C=O stretching present in ketene. |
|
|
2091 |
N=C=S stretching present in isothiocyanate. |
|
|
1635 |
A lot of result. |
|
|
Plant extract sample 2 |
3337 |
O-H present in phenols. |
|
2193 |
C=C=O stretching present in ketene. |
|
|
2091 |
N=C=S stretching present in isothiocyanate. |
|
|
1990 |
A lot of result. |
|
|
1636 |
A lot of result. |
|
|
Plant extract sample 3 |
3337 |
O-H present in phenols. |
|
2215 |
C≡C stretching present in alkyne. |
|
|
2094 |
N=C=S stretching present in isothiocyanate. |
|
|
1636 |
A lot of result. |
|
|
Soil solution sample 1 |
3337 |
O-H present in phenols. |
|
2177 |
C=C=O stretching present in ketene. |
|
|
2104 |
C≡C stretching present in alkyne. |
|
|
1987 |
C=C=C stretching present in allene. |
|
|
1974 |
C=C=C stretching present in allene. |
|
|
1636 |
A lot of result. |
|
|
Soil solution sample 2 |
3306 |
O-H present in phenols. |
|
2184 |
C=C=O stretching present in ketene. |
|
|
2108 |
C≡C stretching present in alkyne. |
|
|
1636 |
A lot of result. |
|
|
Soil solution sample 3 |
3336 |
O-H present in phenols. |
|
2217 |
C≡C stretching present in alkyne. |
|
|
2152 |
N=N=N stretching present in azide. |
|
|
2116 |
C=C=O stretching present in ketene. |
|
|
1637 |
A lot of result. |
Table 2: Assignment of IR absorption bands in the spectra of the Prosopis juliflora.
4. Conclusions
Prosopis juliflora, an invasive plant species, poses significant ecological threats through allelopathy, which involves releasing chemicals that inhibit other plants. FTIR spectroscopy has identified various chemical groups in P. juliflora's extracts and soil solutions, such as nitriles, alkynes, and carbonyls, which impair plant growth by disrupting cellular functions. These chemicals negatively impact the biodiversity of affected areas by reducing species richness and altering plant communities. The potential biodiversity loss due to this invasive species is particularly alarming in regions like Saudi Arabia, where it threatens native flora and ecosystem health. Immediate measures are necessary to control its spread and protect biodiversity and ecosystem services.
Acknowledgements
The authors extend their appreciation to the Ongoing Research Funding Program (ORF-2025-558), King Saud University, Riyadh, Saudi Arabia.
Conflict of Interest
The authors declare no conflict of interest.
References
- Slate ML, Tsombou FM, Callaway RM, et al. Exotic Prosopis juliflora suppresses understory diversity and promotes agricultural weeds more than a native congener. Plant Ecology 221 (2020): 659-669
- Simberloff D, Martin JL, Genovesi P, et al. Impacts of biological invasions: What’s what and the way forward. In Trends in Ecology and Evolution 28 (2013): 58-66.
- Vila M, Espinar JL, Hejda M, et al. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecology Letters 14 (2011): 702-708.
- Besaw LM, Thelen GC, Sutherland S, et al. Disturbance, resource pulses and invasion: Short-term shifts in competitive effects, not growth responses, favour exotic annuals. Journal of Applied Ecology 48 (2011): 998-1006.
- Bibi S, Bibi A, Al-Ghouti MA, et al. Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems. Agronomy 13 (2023): 590.
- Shiferaw H, Bewket W, Alamirew T, et al. Impacts of Prosopis juliflora invasion on livelihoods and ecosystem services in Afar region, Ethiopia. Journal of Arid Environments 163 (2019): 94-104.
- Shiferaw W, Demissew S. Effects of the invasive alien Prosopis juliflora (sw.) DC and its management options in Ethiopia: a review. Tropical Plant Species and Technological Interventions for Improvement (2022).
- Kaur R, Mehra K, Balachandar D. Invasion of Prosopis juliflora in Indian drylands: An ecohydrological perspective. International Journal of Ecology and Environmental Sciences 38 (2012): 75-84.
- El-Keblawy A, Al-Rawai A. Impacts of the invasive exotic Prosopis juliflora (Sw.) DC on the native flora and soils of the UAE. Plant Ecology 190 (2007): 23-35.
- Al Musalami AA, Al Marshoudi MS, Farooq SA, et al. Allelopathic effects of the invasive species (Prosopis juliflora) on seedlings of two common arid plants: Does free proline play roles?. Journal of Arid Environments 211 (2023): 104931.
- Shan Z, Zhou S, Shah A, et al. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 13 (2023): 13092358.
- Inderjit, Dakshini KMM. Allelopathic potential of a weed, Prosopis juliflora (Sw.) DC. Journal of Chemical Ecology 20 (1994): 3221-3232.
- Pandey CB, Singh AK, Saha D, et al. Prosopis juliflora (Swartz) DC.: an invasive alien in community grazing lands and its control through utilization in the Indian Thar Desert. Arid Land Research and Management 33 (2019): 427-448.
- Ayub M, Shehzad M, Siddique MS, et al. Allelopathic effect of legumes leachates on seed germination and seedling growth of maize (Zea mays L.). Journal of Agricultural Technology 9 (2013): 863-875.
- Abdulahi MM, Ute JA, Regasa T. Prosopis Juliflora l: Distribution, impacts and available control methods in Ethiopia. In Tropical and Subtropical Agroecosystems. Universidad Autonoma de Yucatan 20 (2017): 75-89.
- Sirmah PK. Towards valorisation of Prosopis juliflora as an alternative to the declining wood resource in Kenya (Doctoral dissertation, Doctoral dissertation, Ph. D. Dissertation. Universite Henri Poincare, Nancy, France (2009): 30-31.
- Singh S. Phytochemical analysis and localization of bioactive compounds in Prosopis juliflora (Doctoral dissertation, BITS, Pilani (2012).
- Baseri MK, Baker S. Identification of cellular components of medicinal plants using FTIR. Rom. J Biophys 21 (2011): 277-284.
- Bolade OP, Akinsiku AA, Adeyemi AO, et al. Qualitative analysis, total phenolic content, FT-IR and GC-MS characterisation of Canna indica: bioreducing agent for nanoparticles synthesis. In Journal of Physics: Conference Series, IOP Publishing 1299 (2019): 012135).
- Straková P, Larmola T, Andrés J, et al. Quantification of plant root species composition in peatlands using FTIR spectroscopy. Frontiers in plant science 11 (2020): 597.
- Luo Y, Du Z, Yan Z, et al. Artemisia halodendron Litters Have Strong Negative Allelopathic Effects on Earlier Successional Plants in a SemiArid Sandy Dune Region in China. Front. Plant Sci 11 (2020): 961.
- Ali HE, Al-Wahaibi AM, Shahid MS. Plant–soil feedback and plant invasion: effect (2024).
- De Long JR, Heinen R, Heinze J, et al. Plant-soil feedback: incorporating untested influential drivers and reconciling terminology. Plant Soil 485 (2023): 7-43.
- Ferber E, Gerhards J, Sauer M, et al. Chemical Priming by Isothiocyanates Protects Against Intoxication by Products of the Mustard Oil Bomb. Front. Plant Sci 11 (2020): 887.
- Liu Z, Wang Y, Liu K, et al. Integrated Cobaloxime and Mesoporous Silica-Supported Ruthenium/Diamine Co-Catalysis for One-Pot Hydration/Reduction Enantioselective Sequential Reaction of Alkynes. Front Chem 9 (2021): 732542.
- Blackburn TM, Pysek P, Bacher S, et al. A proposed unified framework for biological invasions. Trends Ecol. Evol 26 (2011): 333-339.
- Cuda J, Skalova H, Janovsky Z, et al. Competition among native and invasive Impatiens species: the roles of environmental factors, population density and life stage. AoB Plants 7 (2015): plv033.
- Calderón R, Jara C, Albornoz F, et al. Exploring the destiny and distribution of thiocyanate in the water-soil-plant system and the potential impacts on human health. Science of The Total Environment 835 (2022): 155502.
- Tian P, Feng YX, Li YH. SOS! Hydrogen Sulfide Enhances the Flavonoid Early Warning System in Rice Plants to Cope with Thiocyanate Pollution. Toxics 12 (2024): 591.
- Cuellar-Núñez ML, Loarca-Piña G, Berhow M, et al. Glucosinolate-rich hydrolyzed extract from Moringa oleifera leaves decreased the production of TNF-α and IL-1β cytokines and induced ROS and apoptosis in human colon cancer cells. Journal of Functional Foods 75 (2020): 1-13.
- Patil S, Gavandi T, Karuppayil SM, et al. Glucosinolate derivatives as antifungals: A review. Phytotherapy Research (2024).
- Ali HE, Bucher SF. Effect of drought and nutrient availability on invaded plant communities in a semi-arid ecosystem. Ecol Evol 12 (2022): e9296.
- Zhang L, Zhang L, Huang L, et al. Plant-soil feedback of companion species during grassland community succession. Forests 14 (2023): 1634.
- Malajczuk CJ, Armstrong BI, Stachura SS, et al. Mechanisms of interaction of small hydroxylated cryosolvents with dehydrated model cell membranes: Stabilization vs destruction. J Phys Chem B 126 (2022): 197-216.
- Galindo FA, Venzmer J, Prévost S, et al. Incorporation of short-chain alcohols into fluid bilayers and its effect on membrane dynamic properties as seen by neutron scattering, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 702 (2024): 135014.
- Alfagham A, Bahammam N, Alsahli W, et al. Plant diversity in King Salman Park in Riyadh, Saudi Arabia. Applied Ecology & Environmental Research 20 (2022).
- Al-Abdali S, Al-Dhuhli A, Al-Reasi H. Preliminary investigations of allelopathic effects and herbicide-based eradication of Mesquite (Prosopis juliflora). Sultan Qaboos University Journal for Science [SQUJS] 24 (2019): 11-17.
- Chou CH. Allelopathic mechanisms of Arctostaphylos glandulosa var. zacensis. Dissertation Abstracts International B 32 (1972).
- Ali HE, Al-Wahaibi AM, Shahid MS. Effect of soil conditioning on native and invasive Prosopis species using the plant functional trait approach. Front. Plant Sci 15 (2024): 1321950.
- Zhang YJ, Jin YH, Xu JW, et al. Responses and feedback of litter properties and soil mesofauna to herbaceous plants expansion into the alpine tundra on Changbai Mountain, China. J. Mountain Sci 19 (2022): 403-417.