Culture Positive Cases of Enteric Fever and Their Antibiotic Susceptibility Patterns in a Tertiary Care Hospital in Dhaka, Bangladesh
Rehana Razzak Khan*1, Sourav Debnath1, SM Ali Ahmed1, A S M Nowroz2, Chandan Kumar Roy1, Ismet Nigar1, Shaila Akhtar3, Shaheda Anwar1
1Department of Microbiology and Immunology, Bangladesh Medical University, Dhaka, Bangladesh
2Department of Laboratory Medicine, Bangladesh Medical University, Dhaka, Bangladesh
3Department of Microbiology, Green Life Medical College, Dhaka, Bangladesh
*Corresponding author: Rehana Razzak Khan, Department of Microbiology and Immunology, Bangladesh Medical University, Dhaka, Bangladesh.
Received: 18 June 2025; Accepted: 23 May 2025; Published: 30 June 2025
Article Information
Citation: Rehana Razzak Khan, Sourav Debnath, SM Ali Ahmed, A S M Nowroz, Chandan Kumar Roy, Ismet Nigar, Shaila Akhtar, Shaheda Anwar. Culture Positive Cases of Enteric Fever and Their Antibiotic Susceptibility Patterns in a Tertiary Care Hospital in Dhaka, Bangladesh. Archives of Microbiology and Immunology. 9 (2025): 214-222.
Share at FacebookAbstract
Background: Enteric fever is a systemic bacterial infection caused by Salmonella enterica subspecies enterica serotype Typhi (S. Typhi) or Paratyphi A, B, or C (Paratyphi A, B, or C). It is a significant public health concern in developing countries. Periodic monitoring of sensitivity and resistance patterns is necessary to support therapeutic care at both national and local levels, given the increasing antibiotic resistance observed in their management. Additionally, this will enable the planning of antibiotic recycling whenever possible.
Objective: This study aimed to identify Salmonella, the most prevalent pathogen in bloodstream infections, as well as to determine their demographics, seasonal variations, and antibiotic susceptibility to create a complete picture of how their antibiotic susceptibility has changed over time.
Methods: This retrospective study was conducted in the Department of Microbiology and Immunology, Bangladesh Medical University (BMU), Dhaka, between July 2023 and June 2024. Ten thousand, fifty-three blood samples were collected in adult and pediatric Brain Heart Infusion broth (BHI) bottles for culture, and the bacterial profile was retrieved. The collected samples were cultured using a standard technique in a medical microbiology laboratory. The isolated bacteria were identified by colony morphology, Gram staining, and biochemical reaction. Antibiotic susceptibility was tested by Kirby-Bauer disc diffusion methods per the National Committee for Clinical Laboratory Standards guidelines.
Results: A total of 10053 blood samples were collected, of which 513(5.10%) were culture positive. Among 513 culture growth, the prevailing isolate was Salmonella Typhi 216 (42.10%), followed by Staphylococcus aureus 81(15.80%), Salmonella Paratyphi A 60 (11.70%). Following that, Acinetobacter spp. (10.90%), Klebsiella spp. (9.90%), Pseudomonas spp. (4.30%), E. coli (3.30%), Serratia spp. (0.97%), Burkholderia cepacia (0.60%), Enterobacter spp., and Stenotrophomonas maltophilia (0.39%). The majority of blood culture-positive cases (S. Typhi or S. Paratyphi A) were below 10 years old, with male predominance. There were two surges of enteric fever, the highest prevalence in October through January and March through May. It was found that S. Typhi (13%–19.4 %) and S. Paratyphi A (10- 11.7%) had decreasing resistance to the first-line medications amoxicillin, chloramphenicol, and cotrimoxazole. In cases of enteric fever, the majority of isolates exhibited fluoroquinolone resistance, but none of them exhibited ceftriaxone resistance. There is an increase in azithromycin resistance (85.2%).
Conclusion: The findings of this study highlight the declining resistance to first-line antimicrobials, and it could be reintroduced as an empirical treatment option for enteric fever. Antibiotic use without prescription should be minimized, and prescribing practice should be modified. These results underscore the necessity for judicious use of antimicrobials and the implementation of an antimicrobial stewardship program in tertiary care hospitals across Bangladesh.
Keywords
Enteric fever, antibiotic susceptibility, BHI
Enteric fever articles, antibiotic susceptibility articles, BHI articles
Article Details
Introduction
Karl Joseph Eberth initially described the bacillus thought to cause typhoid in 1980, and four years later, pathologist Georg Graffky confirmed these findings. As a consequence, the bacillus was given the name Eberthella typhi, which is now more commonly referred to as Salmonella enterica [1]. A major public health concern in developing countries is enteric fever [2], which is brought on by Salmonella enterica subspecies enterica serotype Typhi (S. Typhi) or Paratyphi A, B, or C [3]. According to the World Health Organization (WHO), there are between 11 and 20 million typhoid infections worldwide each year, with a mortality rate of 128,000 to 161,000 deaths [4]. Bangladesh is one of the nations that are badly afflicted, with an annual incidence rate of 252 per 100,000 affected [5]. The most cases occurred among children aged 5–9 years; 56% were in individuals under <15 years [3]. Under five-year-olds, who are particularly immunocompromised [6], are significantly more susceptible than older people [7]. Lacking clean water, food, and inadequate sanitation in poor communities are also prone [4,6]. According to South Asia's overall sex discrepancies in enteric fever reporting, men made up the majority of cases (59%) [8]. In many Asian and African nations, enteric fever frequently recurs around the same time of year and exhibits a seasonal pattern [9]. The seasonal pattern of enteric fever in North America and Europe indicated that August to September was the peak time. The peak seasons in Asia, Africa, and the Middle East lasted for several months. Africa and the Middle East from July to November, while Asia is from May to October. Due to the Southern Hemisphere's distinct seasonal scheduling, South America's peak season lasted from January to May [10], suggesting that their transmission may be influenced by meteorological conditions [11]. In Bangladesh, Nepal, and Cambodia (South and Southeast Asia), incidence peaks around May to October [9], whereas it manifests in a variety of seasonal rhythms throughout Africa. A clear seasonal cycle with a peak in March–June after the rainy season was noted in Blantyre, Malawi [12]; however, Nairobi, Kenya, revealed more intricate dynamics [13]. The disease has been reported to occur more frequently in Cameroon and the Democratic Republic of the Congo [14].
Geographical and temporal variations affect the distribution of Salmonella species and the pattern of antibiotic resistance [15,16]. South Asia is particularly vulnerable in terms of the emergence and spread of antimicrobial resistance [17]. This harms clinical outcomes [18]. Public health is seriously threatened by antimicrobial resistance (AMR), which was expected to have contributed to 1.27 million deaths in 2019 [19]. Deaths from AMR infections could increase tenfold by 2050 if left unchecked because of the extensive use of antibiotics and a paucity of new antimicrobials [20, 21]. The burden of morbidity from infectious diseases in poor countries is primarily caused by enteric fevers. Due to the growing antibiotic resistance seen in their management, Salmonella and its many species need to be regularly assessed for sensitivity and resistance patterns in order to guide treatment at the local level [22]. Our study aimed to ascertain demographic information and seasonal fluctuation and monitor antibiotic sensitivity patterns that are therapeutically significant because studies from different countries have shown a fluctuating pattern of susceptibility to conventional medications. This will allow for the recycling of antibiotics wherever possible and provide appropriate treatment guidance.
Materials and Methods
Study design
A retrospective review of the laboratory data of blood samples obtained from the inpatient and outpatient departments of Bangladesh Medical University (BMU), Dhaka, between July 2023 and June 2024 was carried out by the Department of Microbiology and Immunology.
Study population
A total of 10053 conventional blood samples were collected during that period. Reports of Salmonella infection from blood cultures were retrieved and analyzed. A comprehensive dataset regarding demographic data, seasonal variation, laboratory results of bacterial isolation, and susceptibility patterns was collected from the Laboratory specimen logbooks using the standard data collection form.
Laboratory procedures
Sample collection
All the samples were aseptically collected in adult and pediatric Brain Heart Infusion (BHI) broth bottles. About 5 -10 ml of blood/bottle for adults and 2-5 ml of blood/bottle for pediatric patients was collected in the blood culture bottle, labeled properly, and transported to the Microbiology laboratory without delay for the bacteriological examination.
Organism isolation and antimicrobial susceptibility
Incubation of the blood in BHI broth lasted 18–24 hours at 37°C, followed by a 7-day observation period. Following 18–24 hours of incubation, blind subculture onto Blood agar and MacConkey agar, and then every other day until day 7. Daily check the bottle of BHI broth for any obvious growth indicators, such as turbidity, gas generation, pellicle development, clotting, hemolysis, etc. The inoculated cultures were incubated overnight for 24 to 48 hours at 37 degrees Celsius. Organisms were identified based on morphology, culture characteristics, and biochemical reactions according to standard microbiological techniques. All the isolates were tested for antimicrobial susceptibility on Muller Hinton Agar (HI Media, India) by the Kirby Bauer disc diffusion method, according to the Clinical Laboratory Standard Institute (CLSI) guidelines [23]. The following antibiotics were used for Salmonella spp.: amoxicillin (10µg), ciprofloxacin (5µg), nalidixic acid (30µg), ceftriaxone (30µg), trimethoprim-sulfamethoxazole (1.25/23.75µg). And additional azithromycin (30µg) for Salmonella Typhi. All the antibiotic disks were commercially purchased from Biomaxima, Poland. Salmonella Typhimurium ATCC 14028 was included as a quality control strain for antimicrobial susceptibility testing.
Data analysis
Data were cleaned manually, entered, and analyzed by using SPSS version 24 software. The statistical analysis used in the study was descriptive and involved categorical data analysis. Frequency and percentage were examined for categorical independent variables. Results were presented through graphs and tables.
Results
A total of 10053 conventional blood samples were collected, of which 513(5.10%) yielded bacterial growth (Table 1).
Table 1: Frequency of Bacterial isolates in blood sample (n=10053)
|
Culture |
Frequency |
Percentage (%) |
|
Growth |
513 |
5.1 |
|
No Growth |
9540 |
94.89 |
|
Total |
10053 |
100 |
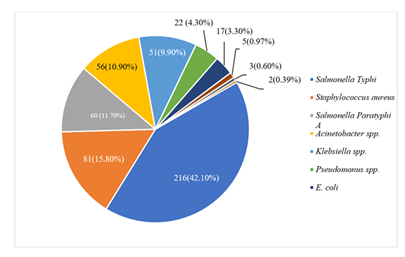
In the current observation, among 513 culture positive cases 216 (42.10%) were caused by Salmonella Typhi, with Staphylococcus aureus 81 (15.80%) and Salmonella Paratyphi A 60 (11.70%) following closely behind Acinetobacter spp., Klebsiella spp., and Pseudomonas spp. were then found in the following proportions: 56 (10.90%), 51 (9.90%), and 22 (4.30%). Lastly, E. coli 17 (3.30%), Serratia spp. 5 (0.97%), and strains of Burkholderia cepacia 3 (0.60%) were identified. Stenotrophomonas maltophilia and Enterobacter spp. were the other two species detected (0.39%) (Figure 1).
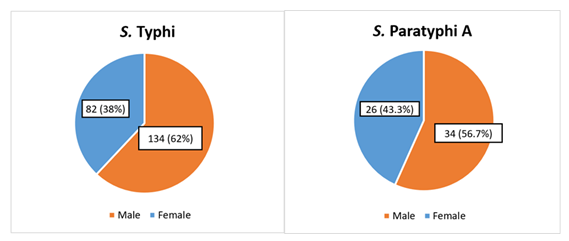
Males (134,62%) were more affected than females (82,38%) in the current study's culture-positive Salmonella Typhi cases. Males made up (34,56.7%) of Salmonella Paratyphi A, while females made up (26.43.3%), showing male preponderance, and male to female ratios were 1.6:1 and 1.3:1, respectively (Figure 2).
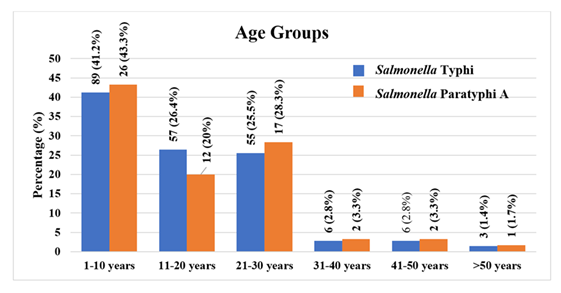
With a positive culture for Salmonella Typhi, the current study found that nearly half (89, 41.2%) of the 216 patients were under 10 years old, followed by those who were 11 to 20 years old (57, 26.4%), 21 to 30 years old (55, 25.5%), 31 to 40 years old (6, 2.8%), 41 to 50 years old (6, 2.8%), and only (3, 1.4%) older than 50. Nearly half of the 60 Salmonella Paratyphi A patients (26, 43.3%) were under ten years old. Only one person was older than 50 (1, 1.7%), followed by those between the ages of 11 and 20 (12, 20%), 21 and 30 (17, 28.3%), 31 and 40 (2, 3.3%), and 41 and 50 (2, 3.3%) (Figure 3).
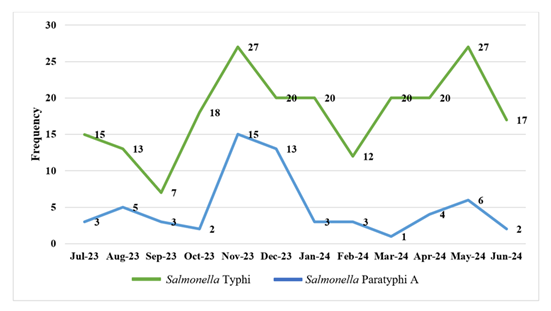
Both cases exhibit a nearly identical seasonal pattern, with the highest number of cases occurring from late monsoon to mid-winter (October to January), followed by the summer season (March to May). Figure 4 provides an illustration of this.
Table 2. Antibiotic resistance pattern of Salmonella Typhi isolated from blood culture.
Resistance to the first-line antimicrobials- amoxicillin, chloramphenicol, cotrimoxazole
We observed the lowest resistance among S. Typhi isolates to first-line antimicrobials amoxicillin (33, 15.3%), chloramphenicol (28,13.0%), trimethoprim-sulfamethoxazole (cotrimoxazole) (42, 19.4%).
Resistance to fluoroquinolone (nalidixic acid, ciprofloxacin) and macrolide (azithromycin)
Non-susceptibility to nalidixic acid (210, 97.2%) and ciprofloxacin (160, 74.1%) in S. Typhi was notably high. And azithromycin was (184, 85.2%) resistant.
Resistance to third-generation cephalosporin - ceftriaxone
None of the S. Typhi isolates were resistant to ceftriaxone.
Table 3: Antibiotic resistance pattern of Salmonella Paratyphi A isolated from blood culture.
Table 2: Antibiotic resistance pattern of Salmonella Typhi to different antibiotics (n=216)
|
Antibiotic Class |
Antibiotics |
Sensitive |
Resistance |
Percentage of Resistance (%) |
|
Penicillin |
Amoxicillin |
183 |
33 |
15.3 (%) |
|
Amphenicol |
Chloramphenicol |
182 |
28 |
13.0 (%) |
|
Sulfonamides |
Trimethoprim-Sulfamethoxazole |
174 |
42 |
19.4 (%) |
|
Cephalosporin |
Ceftriaxone |
216 |
0 |
0 (%) |
|
Fluoroquinolones |
Nalidixic acid |
6 |
210 |
97.2 (%) |
|
Ciprofloxacin |
50 |
160 |
74.1 (%) |
|
|
Macrolide |
Azithromycin |
32 |
184 |
85.20% |
Resistance to the first-line antimicrobials- amoxicillin, chloramphenicol, cotrimoxazole
Declining resistance was displayed among S. Paratyphi A isolates to first-line antimicrobials amoxicillin (7,11.7%), chloramphenicol (6,10%), trimethoprim-sulfamethoxazole (cotrimoxazole) (6, 10%).
Resistance to fluoroquinolone (nalidixic acid, ciprofloxacin) and ceftriaxone
Non-susceptibility to nalidixic acid (50, 83.3%) and ciprofloxacin (52, 86.7%) in S. Paratyphi A was also alarming. While ceftriaxone showed 100% sensitivity.
Table 3: Antibiotic resistance pattern of Salmonella Paratyphi A to different antibiotics (n=60)
|
Antibiotic Class |
Antibiotics |
Sensitive |
Resistance |
Percentage of Resistance (%) |
|
Penicillin |
Amoxicillin |
53 |
7 |
11.7 (%) |
|
Amphenicol |
Chloramphenicol |
54 |
6 |
10 (%) |
|
Sulfonamides |
Trimethoprim-Sulfamethoxazole |
54 |
6 |
10 (%) |
|
Cephalosporin |
Ceftriaxone |
60 |
0 |
0 (%) |
|
Fluoroquinolones |
Nalidixic acid |
10 |
50 |
83.3 (%) |
|
Ciprofloxacin |
8 |
52 |
86.7 (%) |
Discussion
Bangladesh is one of the developing nations where enteric fever is still a major health concern. S. Typhi bacteremia accounts for the majority of these instances, while S. Paratyphi A bacteremia accounts for the remaining occurrences. The bacterial isolation rate in this study was 5.10%, comparable to the 3% in Bangladesh and 0.53%, 4.1%, and 2.2% in India, Nepal, and Pakistan, respectively, reported in Barkume et al.'s (2018) multiphase surveillance study for the Surveillance for Enteric Fever in Asia Pacific (SEAP) [8]. However, Nasin et al. (2021) reported a 13% isolation rate [24]. Additionally, 10-year retrospective research conducted in Bangladesh found that the isolation rate ranged from 10.7 to 17.3% [25]. These differences in the bacterial isolation rate could be due to the different institutions’ employment of varied sample collection techniques, culture techniques, and infection control protocols. Salmonella Typhi (42.10%) was the most common isolate in the present study, followed by Staphylococcus aureus (15.80%) and Salmonella Paratyphi A (11.70%). Salmonella Paratyphi B was not found in any of the isolates during our analysis. Numerous studies carried out in Bangladesh have found that Salmonella Typhi is a frequent cause of bloodstream infections in this region [7,26–27]. According to Nasrin et al. (51%) were S. Typhi, and (15%) were S. Paratyphi A, which is almost consistent with our study, and 6 percent of isolates were Staphylococcus aureus [24]. Ahmed et al. reported S. Typhi (36.9%) and S. Paratyphi A, B (8.9%) [25]. However, Singh et al. demonstrated that Staphylococcus aureus (33.10%) was their most common isolate [22], nearly twice as high as our study. The culture, technique, and infection control policies of each unique institution are the cause of this discrepancy.
However, according to Date et al. (2016), the first thorough analysis of National Typhoid and Paratyphoid Fever Surveillance System (NTPFS) data over a five-year period, 80% of cases in the United States were typhoid, and 20% were paratyphoid A [28]. This discrepancy results from the fact that an increasing proportion of cases have been linked to travelers returning from Southern Asia [2]. In addition, Acinetobacter species, Klebsiella species, and Pseudomonas species were isolated in this study; their respective percentages were (10.90%), (9.90%), and (4.30%). Finally, it was found that E. coli (3.30%), Serratia spp. (0.97%), and Burkholderia cepacia (0.6%) were present. Two more organisms, Stenotrophomonas maltophilia and Enterobacter spp., were found (0.39%). This is nearly in line with the Singh et al. study, which found that Acinetobacter species accounted for 10.61%, Klebsiella species for 7.69%, and Pseudomonas species for 6.42% [22]. However, E. coli levels in other studies were slightly higher than ours (12.07% - 15%) [22, 24]. Ahmed et al found Pseudomonas species (12.5%) and Acinetobacter species (5.1%); besides this, E. coli, Enterobacter spp., and Serratia species had a steady isolation rate over the ten years, while Klebsiella species showed an increasing trend in their isolation rate from 22.6 to 50.9% [25]. The rise in BSI from different organisms may have resulted from patients' greater access to medical services at that time in their study period [25]. The reason for this difference from our study is due to the organisms' shifting patterns.
In this study, we found that males were predominantly suffering from enteric fever than females, which is in line with the typical sex differences in enteric fever reporting in South Asia [8]. These results show a significant degree of sex-specific variance in illness, even though Zabeen et al. and Mina et al. reported a female predominance [29,30]. Whether any factors contribute to the sex-specific vulnerability of enteric fever patients can be ascertained by additional research. In any microbiological infection, the patient's age is always a significant factor. Enteric fever can infect people of any age [30]. The majority of the S. Typhi and S. Paratyphi A culture-positive patients in our study were from the age group below 10 years. This finding was consistent with a different study that found children aged 5 to 9 years and under 10 years had the highest incidence [3,13]. Those aged 11-30 years were similarly affected by S. Typhi, whereas the age group of 11–20 years old is less affected than the group of 21–30 years old in S. Paratyphi A culture-positive patients in our study. The previous study represented that the patients infected by S. Typhi were observed under the age of 20 years, though the maximum of them were under the age of 5 [7,8, 30,31]. While Barkume et al. reported, in India and Nepal, a high proportion of participants aged 15-25 years, and in a previous study in Bangladesh and Pakistan were aged ≤ 5 years [8]. Because young children typically suffer from malnutrition, changes in the gut flora or other host defenses may increase susceptibility to reinfection [32]. Evidence from past studies that shows environmental variation in the risk of infection between children and adults is emphasized by Akullian et al. [13]. Also, this infection is brought on by school-age children's unsanitary behavior, poor hygiene practices, or developing a habit of consuming local street foods, as it is more affordable and convenient in a developing nation like Bangladesh. Adults are particularly at risk since they make up the majority of the workforce and engage in more outside activities in poor nations, which allows them to consume cool water and consume undercooked or street cuisine on hot days.
The two positive spikes of enteric fever in the current study occurred in the fall and winter (late monsoon to cool dry winter) months of October through January, and in the summer (pre-monsoon hot season) months of March through May. It is consistent with the study by Jayaprasad et al., who reported the highest case positivity in January 2017[33]. In contrast, several previous studies in which the South Asian case burden was described as highest during periods of peak rainfall or just after, the months of May to October [27,29,10,33]. This shifting pattern of wintertime enteric fever may be brought on by past travel or improper storage of food consumption. Additionally, cold air impairs immunity, making it more difficult to fend off infections. And in summer dry season peak in our study correlate with other study that represent increase temperature would promote the growth and reproduction of Salmonellae, leads to water source pollution, water scarcity, using contaminated water sources, engage in more outdoor recreational activities and encouraging the purchase of prepared foods or barbecues during warmer weather leads to infection [34, 35] In a range of geographical contexts, numerous studies have shown a positive correlation between temperature and foodborne disease like enteric fever [36-38]. In our study, the antibiotic resistance pattern was almost the same for S. Typhi and S. Paratyphi A. Resistance to the first-line antibiotics amoxicillin, chloramphenicol, and cotrimoxazole decreased in S. Typhi (13%–19.4 %) and S. Paratyphi A (10–11.7%), respectively. This result is similar to that of a 24-year retrospective observational study by Tanmoy et al. (2024), in which resistance peaked declined for S. Typhi from 80% to less than 20% for all three first-line antimicrobials, and Ahmed et al. (2017) resistance declines from 61.7 to 23.7% [38,25,8]. Alongside a decline in resistance for S. Paratyphi A was (5-7%) to these first-line antimicrobials [24]. Enteric fever surveillance studies across various countries, including Bangladesh, India, Nepal, Pakistan, Vietnam, Laos, Indonesia, have shown that this fall was linked to a decrease in multidrug resistance (MDR) [38–45]. This decrease in resistance may be the consequence of fewer doctors prescribing first-line antibiotics and a drop in usage, especially of cotrimoxazole. In our present observation, we did not find any MDR Salmonella cases. Whereas Khan et al (2024) in Pakistan showed high resistance rates to ampicillin (81.40%) and chloramphenicol (90.2%), respectively, and did not mention cotrimoxazole, they also mention MDR cases [46]. This difference is due to geographical and environmental variance and a decline in the consumption of these first-line antimicrobials in our country.
Our study found that (85.2%) of Salmonella Typhi were resistant to azithromycin, which is quite concerning. This is in line with the research report, Bangladesh suggests a rising effective population size of azithromycin-resistant isolates [47]. High consumption of the drug may exert selective pressure, fostering resistance determinants in the gut [48]. However, another study highlights the potential of azithromycin as a viable treatment option with a notable (90.40% -93%) sensitivity [46,49,50]. This concurs with the WHO’s recognition of azithromycin as an alternative treatment for MDR typhoid fever, particularly in regions facing high resistance to conventional drugs [51]. We did not test for Salmonella Paratyphi A sensitivity against azithromycin. In the present study, we found remarkable resistance against the fluoroquinolones class of antibiotics, nalidixic acid, and ciprofloxacin in both Salmonella Typhi and Salmonella Paratyphi A. This is consistent with several studies where the majority of isolates in all countries were resistant to fluoroquinolones [8,49,38,46]. It suggests that these medications are being abused and used irrationally to treat many other nonspecific illnesses. In the current study, no isolates showed resistance to ceftriaxone in enteric fever cases; similarly, multiple studies showed 100% sensitivity to ceftriaxone [49]. However, Hooda et al. discovered 47 cases of ceftriaxone-resistant Salmonella Typhi in Bangladesh between April and September 2024, in addition to a few solitary cases that had already been reported in Bangladesh and India [52–54]. Since ceftriaxone empirical treatment is so common in South Asia, public health officials need to keep an eye out for the emergence and spread of ceftriaxone-resistant Salmonella Typhi [55]. Antimicrobial resistance in enteric fever is mostly caused by the inappropriate, excessive, and misused use of antimicrobial drugs. Hence, the result may not reflect the national scenario of this endemic infection; physicians should consider this before prescribing antimicrobials.
Conclusion
According to the current study, children under the age of ten are primarily afflicted by enteric fever, which is endemic throughout the year, with two significant incidence spikes from October to January and another peak from March to May. Third-generation cephalosporins need to be closely monitored, and reintroducing first-line antibiotics to treat this infection is one possible antibiotic stewardship technique that we suggest in order to observe the AMR pattern in enteric fever cases. These results indicate that treatment practices need to change, and immediate action is required to curb antibiotic abuse. It is essential to routinely evaluate the microbiological profile in order to guarantee efficient management of enteric fever cases, especially in light of the expanding problem of medication resistance.
Limitation
Because the study is retrospective, we are unable to distinguish between samples from patients outside of our hospitals and those from our hospitals. Apart from age and sex, we were also unable to gather information about the clinical manifestation of the patient or any other patient features. Clinical connection is therefore not possible to some degree.
Acknowledgement
We thank the entire technical team of the Microbiology Laboratory, Microbiology & Immunology Department, Bangabandhu Medical University.
Conflict of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Authors Contributions
All authors contributed equally to this work.
Funding
This study did not receive any funding.
References
- https://www.news-medical.net/health/Typhoid-Fever-History.aspx.
- Menezes GA, Harish BN, Khan MA, et al. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005-2009.Clin Microbiol Infect 18 (2012): 239-248.
- GBD 2017 Typhoid and Paratyphoid Collaborators, The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19 (2019): 369–381.
- https://www.who.int/news-room/fact-sheets/detail/typhoid. (Accessed 18 June 2022).
- Forster DP, Leder K. Typhoid fever in travelers: estimating the risk of acquisition by country, J Travel Med. 28 (2021): 8.
- Tharwani ZH, Kumar P, Salman Y, et al. Typhoid in Pakistan: Challenges, Efforts, and Recommendations. Infection and Drug Resistance 15 (2022): 2523-2527.
- Naheed A, Ram PK, Brooks WA, Hossain MA, Parsons MB, Talukder KA, et al. Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh. Int J Infect Dis 14 (2010): e93–e99.
- Barkume C, Date K, Saha SK, et al. Phase I of the Surveillance for Enteric Fever in Asia Project (SEAP): an overview and lessons learned. J Infect Dis 218 (2018): S188-S194
- Pham Thanh D, Thompson CN, Rabaa MA, et al. The Molecular and Spatial Epidemiology of Typhoid Fever in Rural Cambodia. PLoS Negl Trop Dis 10 (2016): 1-16.
- Saad NJ, Lynch VD, Antillon M, et al. Seasonal dynamics of typhoid and paratyphoid fever. Scientific Reports 8 (2018): 6870.
- Vollard AM, Ali S, van Asten HAGH, et al. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA 291 (2004): 2607–15.
- Pitzer VE, Feasey NA, Msefula C, et al. Mathematical modeling to assess the drivers of the recent emergence of typhoid fever in Blantyre, Malawi. Clin Infect Dis 61 (2015): 251–258.
- Akullian A, Ng’eno E, Matheson AI, et al. Environmental Transmission of Typhoid Fever in an Urban Slum. PLoS Negl Trop Dis 9 (2015): e0004212.
- Ako AA, Nkeng GE, Takem G E. Water quality and occurrence of water-borne diseases in the Douala 4th District, Cameroon. Water Sci Technol 59 (2009): 2321–2329.
- Iyer RN, Jangam RR, Jacinth A, et al. Prevalence and trends in the antimicrobial susceptibility pattern of Salmonella enterica serovars Typhi and Paratyphi A among children in a pediatric tertiary care hospital in South India over a period of ten years: a retrospective study. Eur J Clin Microbiol Infect Dis 36 (2017): 2399 -2404.
- John J, Van Aart CJ, Grassly NC. The burden of typhoid and paratyphoid in India: systematic review and meta-analysis. PLoS Negl Trop Dis 10 (2016): e0004616.
- Chereau F, Opatowski L, Tourdjman M, et al. Risk assessment for antibiotic resistance in South East Asia. BMJ 358 (2017): 2–8.
- Kern WV, Rieg S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug resistant pathogens. Clin Microbiol Infect 26 (2020):151–157.
- Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399 (2022):629–655.
- Atkins KE, Flasche S. Vaccination to reduce antimicrobial resistance. The Lancet Glob Health. 6 (2018): e252.
- Baker RE, Mahmud AS, Miller IF, et al. Infectious disease in an era of global change. Nat Rev Microbiol 20 (2022): 193-205.
- Singh L, Cariappa MP. Blood culture isolates and antibiogram of Salmonella: Experience of a tertiary care hospital. Med J Armed Forces India 72 (2016): 281-284.
- Clinical Laboratory Standard Institute (CLSI) performance standards for antimicrobial susceptibility testing.33ED. CLSI supplement M100 B Wayne. PA: Clinical and Laboratory Institute (2023).
- Nasrin M, Begum FM, Karim R, et al. Bacteriological profile and antimicrobial susceptibility patterns of blood culture isolates among bloodstream infection suspected patients attending in a referral hospital. Bangladesh J Med Microbiol 15 (2021): 5-11
- Ahmed D, Nahid MA, Sami AB, et al. Bacterial etiology of bloodstream infections and antimicrobial resistance in Dhaka, Bangladesh 2005-2014. Antimicrobial Resistance and Infection Control 6 (2017): 2.
- Brooks WA, Hossain A, Goswami D, et al. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis 11 (2005): 326–329.
- Saha SK, Baqui AH, Hanif M, et al. Typhoid fever in Bangladesh: implications for vaccination policy. The Pediatric infectious disease journal 20 (2001): 521-524.
- Date KA, Newtoon AE, Medalla F, et al. Changing Patterns in Enteric Fever Incidence and Increasing Antibiotic Resistance of Enteric Fever Isolates in the United States, 2008-2012.Clin Infect Dis 63 (2016): 322-329.
- Zabeen F, Hasan MQ, Farheen C, et al. Socio-Demographic Characteristics and Clinical Profiles of Children with Enteric Fever: Experience at a Tertiary Care Hospital of Bangladesh. Bangladesh Journal of Infectious Diseases. Dec 8 (2021): 75-81.
- Mina SA, Hasan MZ, Hossain AKM, et al. The Prevalence of Multi-Drug-Resistant Salmonella Typhi isolated From Blood Sample. SAGE 16 (2023).
- Khanam F, Sayeed MA, Choudhury FK, et al. Typhoid fever in young children in Bangladesh: clinical findings, antibiotic susceptibility pattern and immune responses. PLoS Negl Trop Dis 9 (2015): e0003619.
- Zaki SA, Karande S. Multidrug-resistant typhoid fever: a review. J Infect Dev Ctries 5 (2011): 324-337.
- Jayaprasad N, Borhade P, LeBoa C, et al. Retrospective Review of Blood Culture -Confirmed Cases of Enteric fever in Navi Mumbai, India: 2014-2018. Am J Trop Med Hyg 109 (2023): 571-574.
- Baracchini T, King AA, Bouma MJ, et al. Seasonality in cholera dynamics: a rainfall-driven model explains the wide range of patterns in endemic areas. Adv Water Resour 108 (2017): 357–366.
- Lake I, Gillespie I, Bentham G, et al. A reevaluation of the impact of temperature and climate change on foodborne illness. Epidemiol Infect 137 (2009): 1538–47.
- Bentham G, Langford IH. Environmental temperatures and the incidence of food poisoning in England and Wales. Int J Biometeorol 45 (2001): 22–26.
- Kovats R, Edwards S, Hajat S, et al. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect 132 (2004): 443–453.
- Tanmoy AM, Hooda Y, Sajib MSI, et al. Trends in antimicrobial resistance amongst Salmonella Typhi in Bangladesh: A 24- year retrospective observational study (1999-2022). PLoS Negl Trop Dis 18 (2024): e0012558.
- Carey ME, Dyson ZA, Ingle DJ, et al. Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes. e Life 12 (2023): e85867.
- Balaji V, Kapil A, Shastri J, et al. Longitudinal Typhoid Fever Trends in India from 2000 to 2015. Am J Trop Med Hyg 99 (2018): 34–40.
- Khadka S, Shrestha B, Pokhrel A, et al. Antimicrobial Resistance in Salmonella Typhi Isolated from a Referral Hospital of Kathmandu, Nepal. Microbiology Insights 14 (2021): 11786361211056350.
- Qamar FN, Yousafzai MT, Sultana S, et al. A Retrospective Study of Laboratory-Based Enteric Fever Surveillance, Pakistan, 2012–2014. J Infect Dis 218 (2018): S201–S205.
- Nga TVT, Duy PT, Lan NPH, et al. The control of typhoid fever in Vietnam. Am J Trop Med Hyg 99 (2018): 72-78.
- Roberts T, Rattanavong S, Phommasone K, et al. Typhoid in Laos: an 18-year perspective. Am J Trop Med Hyg 102 (2020): 749.
- Jamilah J, Hatta M, Natzir R, et al. Analysis of existence of multidrug-resistant H58 gene in Salmonella enterica serovar Typhi isolated from typhoid fever patients in Makassar, Indonesia. New Microbes New Infect 38 (2020): 100793.
- Khan Z, Khan AM, Basit A, et al. Pattern of antibiotic sensitivity in typhoid fever patients with possible with positive blood culture: an observational study. Biol. Clin. Sci. Res. J 660 (2024).
- da Silva KE, Tanmoy AM, Pragasam AK, et al. The international and intercontinental spread and expansion of antimicrobial-resistant Salmonella Typhi: a genomic epidemiology study. The Lancet Microbe 3 (2022): e567-e577.
- Doan T, Worden L, Hinterwirth A, et al. Macrolide and Non macrolide Resistance with Mass Azithromycin Distribution. New England Journal of Medicine 383 (2020): 1941–50.
- Manwani DS, Ahirwar DSK, Mutha DA. Comparison of automated and conventional blood cultures and their antibiotic resistance patterns in Salmonella enterica Typhi serovar isolates in a tertiary care Centre. Afr. J. Bio. SC 6 (2024): 351-357.
- Poudel S, Shrestha SK, Pradhan A, et al. Antimicrobial susceptibility pattern Salmonella enteric species in blood culture isolates. Clin Microbial 3 (2014): 342-349.
- Qayyum W, Yousafzai ZA, Afridi M, et al. Drug Resistance Pattern of Salmonella Typhi in Patients Suffering from Enteric Fever-Experience from Tertiary Care Hospital of Peshawar. J Saidu Med Coll Swat 13 (2023): 168-174.
- Hooda Y, Tonmoy AM, Nath SD, et al. Investigation of an ongoing outbreak of ceftriaxone-resistant Salmonella enterica serovar Typhi in Bangladesh. Med Rxiv (2024).
- Djughout B, Saha S, Sajib MSI, et al. Ceftriaxone resistant Salmonella Typhi carries an IncI1-ST31 plasmid encoding CTX-M-15. J Med Microbiol 67 (2018): 620 627.
- Argimón S, Nagaraj G, Shamanna V, et al. Circulation of Third-Generation Cephalosporin Resistant Salmonella Typhi in Mumbai. India. Clin Infect Dis 74 (2022): 2234-2237.
- Hooda Y, Tanmoy AM, Sajib MSI, et al. Mass azithromycin administration: consideration in an increasingly resistant world. BMJ Global Health 5 (2020): e002446.