Dynamics of Erythropoietin Deficiency During the Progression of Chronic Kidney Disease: An Analysis from Stages I to V in Cameroonian Adults
Elimby Ngande Lionel Patrick Joel1,5*, Nguea Ndjame Arlette2,4 and Fouda Menye Epouse Ebana Hermine Danielle1,3
1Faculty of Medicine and Biomedical Sciences, the University of Yaounde I, Cameroon
2Faculty of Medicine and Pharmaceutical Sciences, the University of Douala, Cameroon
3General Douala Hospital, Cameroon
4Yaounde Central Hospital, Cameroon
5Yaounde University Teaching Hospital, Cameroon
*Corresponding Author: Dr. Elimby Ngande Lionel Patrick Joel / Nephrologist / Assistant Lecturer, Faculty of Medicine and Biomedical Sciences, the University of Yaounde I, Yaounde University Teaching Hospital, Cameroon.
Received: 28 June 2025; Accepted: 11 July 2025; Published: 31 July 2025.
Article Information
Citation: Elimby Ngande Lionel Patrick Joel, Nguea Ndjame Arlette and Fouda Menye Epouse Ebana Hermine Danielle. Dynamics of Erythropoietin Deficiency During the Progression of Chronic Kidney Disease: An Analysis from Stages I to V in Cameroonian Adults. Archives of Nephrology and Urology. 8 (2025): 89-93.
DOI: 10.26502/anu.2644-2833101
Share at FacebookAbstract
Background: Erythropoietin deficiency significantly contributes to the development of severe anemia, which in turn leads to therapeutic complications and significantly worsens the prognosis of patients with chronic kidney disease (CKD). Among Cameroonian adults, data on variations in erythropoietin concentrations in CKD patients are scarce. The aim of this study was to explore the dynamics of erythropoietin deficiency during the progression of chronic kidney disease from stages I to V in Cameroonian adults.
Methods: We conducted an analytical cross-sectional study in the nephrology department of the Douala General Hospital over a sixmonth period. A total of 262 patients with CKD at various stages were included. Glomerular filtration rate (GFR), creatinine, and erythropoietin concentrations were measured. One-way analysis of variance (ANOVA) and the Kruskal-Wallis rank sum test were used to compare mean erythropoietin concentrations across CKD stages.
Results: The mean age of the patients was 49 ± 14 years, ranging from 17 to 78 years. The majority were male (60%, n = 157), married (63%, n = 148), and worked in the informal sector (48%, n = 103). Mean erythropoietin levels decreased progressively and significantly across CKD stages: stage I (4.62 ± 2.19 mIU/mL), stage II (3.36 ± 2.06 mIU/mL), stage III (1.86 ± 1.50 mIU/mL), stage IV (0.99 ± 0.58 mIU/mL), and stage V (0.63 ± 0.29 mIU/mL) (P < 0.001).
Conclusion: Our study shows that among Cameroonian adults with CKD, erythropoietin deficiency begins early and progresses from stage II onward, leading to an approximate 86% reduction in serum erythropoietin levels between stages I and V.
Keywords
Erythropoietin deficiency; Chronic kidney disease; Stage; Cameroonian adults
Erythropoietin deficiency articles; Chronic kidney disease articles; Stage articles; Cameroonian adults articles.
Article Details
1. Introduction
CKD (chronic kidney disease) is defined as any functional or structural abnormality of the kidney lasting more than three months, typically identified by a persistent decrease in the glomerular filtration rate (GFR < 60 mL/min/1.73 m²), or by the presence of albuminuria, morphological, or histological abnormalities. Globally, CKD affects over 850 million people—just over 10% of the population—and is now among the leading causes of non-communicable mortality [1]. In Cameroon, available studies report a CKD prevalence among adults ranging between 10% and 14%, primarily driven by hypertension, type 2 diabetes, and HIV infection. Among patients who reach end-stage renal disease and initiate hemodialysis, the one-year mortality rate ranges from 26.8% to 38.6%, underscoring the severity of renal prognosis in this resource-limited setting [2, 3]. Among the complications of CKD, normocytic normochromic anemia holds a central position. A recent meta-analysis estimated its overall prevalence at 42% across all CKD stages, with rates increasing alongside disease severity—up to 80% at stage 5. Anemia worsens fatigue, increases cardiac overload, and raises cardiovascular morbidity and mortality, the leading cause of death in these patients [4]. Pathophysiologically, this anemia mainly results from reduced renal erythropoietin (EPO) production—a phenomenon exacerbated by chronic inflammation, elevated hepcidin levels, and shortened red blood cell lifespan. In adults, serum EPO concentrations decrease as early as stage II and may be over ten times lower than expected for a given level of hypoxia [5, 6]. Since erythropoietin deficiency often precedes the drop in hemoglobin, quantifying serum EPO can help identify patients who may benefit from early initiation of erythropoiesis-stimulating agents, optimize iron stores, and ultimately reduce anemia-related mortality. However, data on EPO dynamics throughout CKD progression remain scarce in Cameroonian adults, highlighting the need for local studies to inform therapeutic strategies. The objective of this study was thus to describe the dynamics of erythropoietin concentrations from stages I to V of CKD in Cameroonian adults, in order to determine when erythropoietin deficiency becomes clinically significant and to support early screening and management strategies for anemia.
2. Methodology
2.1 Study design and setting
We conducted an analytical cross-sectional study over a 6-month period, from August 2024 to January 2025, in the nephrology department of Douala General Hospital, a public facility equipped with a dialysis unit.
2.2 Study population
The study population included all patients with confirmed CKD classified from stage I to stage V according to the KDIGO classification. Patients who consented to participate were included, while those who declined, were concurrently receiving non-steroidal anti-inflammatory drugs or cytotoxic agents, or had acute conditions likely to affect hematopoiesis were excluded. An exhaustive recruitment approach resulted in a final sample of 262 patients, exceeding the minimum required sample size of 169, based on the national prevalence of CKD.
2.3 Data collection
Data were collected using a standardized form covering sociodemographic, clinical, and biological variables (age, sex, occupation, eGFR, creatinine, and serum erythropoietin concentration). A 5 mL venous blood sample was collected from each participant; samples were transported to the laboratory, stored at 2–8°C, and analyzed within 24 hours. Erythropoietin was measured using a sandwich ELISA test, read in duplicate at 450/620 nm, with internal quality control.
2.4 Operational definitions
CKD was defined as persistent renal impairment for at least three months, reflected by an eGFR < 60 mL/min/1.73 m² or by urinary or structural abnormalities. Stages I to V followed the KDIGO thresholds based on eGFR. Erythropoietin deficiency was defined as a concentration below the 5th percentile expected for the degree of anemia or < 2 mIU/mL in stages III to V. Anemia was defined as hemoglobin < 13 g/dL in men or < 12 g/dL in women.
2.5 Statistical analysis
Data were entered in Excel 2016 and analyzed using R version 4.4.2. Quantitative variables were presented as mean ± standard deviation. Comparisons of mean creatinine and EPO concentrations across CKD stages were made using one-way ANOVA or the Kruskal–Wallis test when distributions were non-normal.
2.6 Ethical considerations
The study protocol received approval from the Ethics Committee of Douala General Hospital (Ref. 1713/AR/HGD/SCM/CR). Written informed consent was obtained from each participant. Data were anonymized using alphanumeric codes and stored on a secure server. Residual blood samples were disposed of in accordance with national regulations, and any clinical abnormalities identified were promptly communicated to participants and managed through specialized consultations.
3. Results
3.1 General characteristics of the study population
The mean age of the patients was 49 ± 14 years, with extremes ranging from 17 to 78 years. The majority of patients were male (60%, n = 157). Regarding marital status, married patients were the most represented group (63%, n = 148). In terms of professional sector, patients working in the informal sector accounted for the highest proportion (48%, n = 103). (Table 1).
Table 1: General Characteristics of the Study Population
|
Characteristics |
n(%) |
|
Mean age ± SD [min -max] |
49±14[17-78] |
|
Gender |
|
|
Male |
157 (60%) |
|
Female |
105 (40%) |
|
Marital status |
|
|
Married |
148 (63%) |
|
Single |
67 (29%) |
|
Widowed |
18 (7.7%) |
|
Divorced |
2 (0.9%) |
|
Occupation sector |
|
|
Informal sector |
103 (48%) |
|
Liberal profession |
43 (20%) |
|
Retired |
29 (14%) |
|
Public sector |
15 (7.0%) |
|
Student |
14 (6.5%) |
|
Private sector |
10 (4.7%) |
3.2 Variation in Serum Creatinine and Glomerular Filtration Rate by CKD Stage
The analysis of serum creatinine concentrations and glomerular filtration rate (eGFR) across CKD stages revealed a progressive increase in mean creatinine levels, from 0.80 ± 0.14 mg/dL at stage I to 4.10 ± 0.10 mg/dL at stage V (P < 0.001), and a significant decrease in eGFR, from 94 ± 3 mL/min/1.73 m² at stage I to 12 ± 3 mL/min/1.73 m² at stage V (P < 0.001) (Table 2).
Table 2: Variation in Creatinine Concentrations and Glomerular Filtration Rate Across Chronic Kidney Disease Stages
|
Stage of chronic kidney disease |
||||||
|
stage I N = 16 |
stage II N = 47 |
stage III N = 91 |
stage IV N = 75 |
stage V N = 33 |
p-value |
|
|
Creatinine (mg/dL) |
0.80±0.14a |
1.54±0.39b |
2.98±0.31c |
3.71±0.21d |
4.10±0.10e |
<0.001 |
|
eGFR (mL · min¹ · 1,73 m²) |
94±3a |
77±9b |
41±8c |
23±5d |
12±3e |
<0.001 |
GFR: Glomerular Filtration Rate; data are presented as mean ± standard deviation. P-value, abcde: One-way analysis of variance (ANOVA) was performed to compare the mean values of creatinine concentrations and glomerular filtration rate across the stages. On the same row, means with different letters are statistically different at p < 0.05.
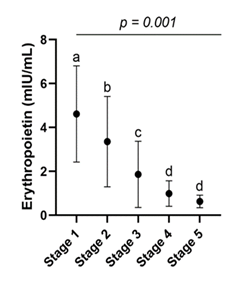
3.3 Variation in Mean Erythropoietin Concentrations by CKD Stage
Figure 1 shows the progression of serum erythropoietin concentrations (in mIU/mL) across the stages of chronic kidney disease. Mean erythropoietin levels decreased progressively and significantly with advancing disease stages. Patients at stage I had the highest concentrations (4.62 ± 2.19 mIU/mL), followed by those at stages II (3.36 ± 2.06 mIU/mL), III (1.86 ± 1.50 mIU/mL), IV (0.99 ± 0.58 mIU/mL), and V (0.63 ± 0.29 mIU/mL). Statistical analysis revealed a significant difference between groups (P < 0.001) (Figure 1).
Figure 1: Evolution of Serum Erythropoietin Concentrations (in mIU/mL) According to Chronic Kidney Disease Stages
4. Discussion
Erythropoietin (EPO) is a glycoprotein produced by peritubular interstitial cells in the renal cortex; in response to hypoxia, it stimulates the proliferation and differentiation of medullary erythroid precursors, thereby maintaining adequate tissue oxygenation [7,8]. In patients with CKD, the progressive destruction of renal parenchyma reduces the functional mass of these cells, resulting in a relative or absolute EPO deficiency. This deficiency is further aggravated by chronic inflammation, disturbances in iron metabolism, hyperparathyroidism, and, at advanced stages, bone marrow resistance to erythropoiesis-stimulating agents [9]. The resulting anemia leads to increased fatigue, cardiac hypertrophy, accelerated progression of CKD, and a poor overall prognosis. Our results showed that mean EPO concentrations steadily decreased from stage I (4.62 mIU/mL) to stage V (0.63 mIU/mL), following an inverse pattern compared to glomerular filtration rate. This decline confirms a failure in endogenous EPO synthesis, which begins early, even with moderate nephron loss. These findings align with international data showing an exponential correlation between eGFR and EPO production [7,10]. At the molecular level, hypoxia induces stabilization of the HIF-α factor; however, interstitial fibrosis, typical of stages III–V, impairs oxygen diffusion and disrupts HIF/EPO signaling. Additionally, urea accumulation and uremic toxins impair hematopoiesis and promote apoptosis of erythroid precursors [9]. This erythropoietic insufficiency explains the severity of anemia observed clinically and supports early use of erythropoiesis-stimulating agents in advanced stages—or more recently, HIF prolyl hydroxylase inhibitors, which have proven effective in increasing hemoglobin levels in non-dialyzed patients [9].
The analysis of sociodemographic characteristics revealed a mean patient age of 49 years, with extremes ranging from 17 to 78 years. The presence of CKD in adolescents may be explained by genetic causes (e.g., high-risk APOL1 variants common in West Africa; CAKUT), post-infectious glomerular nephropathies, early exposure to nephrotoxic phytotoxins, or HIV infection [11]. Recent data indicate that the global burden of CKD among individuals aged 10–24 is steadily rising, particularly in low- and middle-income countries where diagnosis is often delayed [10]. The predominance of male patients (60%) aligns with regional trends and may reflect greater occupational exposure to pesticides, solvents, or heavy metals, as well as lower utilization of preventive healthcare services among men. The high proportion of married patients (63%) highlights the familial impact of CKD, often diagnosed during individuals’ most productive years. Nearly half of the patients worked in the informal sector (48%). This overrepresentation reflects the structure of the Cameroonian economy, where more than 80% of productive units remain unregistered (World Bank, 2024). Informal employment implies unstable income, lack of health insurance, and greater vulnerability to financial shocks. In a country with low universal health coverage, the direct cost of CKD management (USD 163/month for non-dialyzed CKD; > USD 4,000/year for hemodialysis) places a heavy burden on households and contributes to treatment abandonment [12]. Loss of productivity, debt, and secondary impoverishment further diminish quality of life, as highlighted in a recent African review [13]. Our findings provide the first description of the dynamics of erythropoietin deficiency across all CKD stages in an adult Cameroonian population. They underscore the urgent need for early anemia screening in this population. Nevertheless, the cross-sectional design limits causal inference; EPO measurements were not repeated, and iron status and inflammatory biomarkers were not assessed—elements that will be explored in future research.
5. Conclusion
The progressive decline in EPO levels highlights the early onset and rapid worsening of erythropoietic deficiency in CKD. Combined with pronounced socioeconomic vulnerability, these pathophysiological disturbances compromise both the quality of life and prognosis of Cameroonian patients, reinforcing the need to include CKD and its related anemia among the priorities of universal health coverage initiatives.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors state that the research was conducted in the absence of any external funding.
Authors' Contributions
ENLPJ designed the experimental approach and the writing plan. ENLPJ and NNE recruited the participants and conducted the laboratory analyses. ENLPJ performed the statistical analysis and prepared all the figures. ENLPJ drafted the manuscript. ENLPJ, NNA, and FMEHD reviewed the manuscript. All the authors made substantial, direct, and intellectual contributions to the work and approved it for publication.
References
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. avr 12 (2022): 7-11.
- Aseneh JB, Kemah BLA, Mabouna S, et al. Chronic kidney disease in Cameroon: a scoping review. BMC Nephrol 21 (2020): 409.
- Halle MP, Ashuntantang G, Kaze FF, et al. Fatal outcomes among patients on maintenance haemodialysis in sub-Saharan Africa: a 10-year audit from the Douala General Hospital in Cameroon. BMC Nephrol 17 (2016): 165.
- Kim D, Lee J, Toyama T, et al. Prevalence and Treatment Patterns of Anaemia in Individuals With Chronic Kidney Disease Across Asia: A Systematic Review and Meta-Analysis. Nephrol Carlton Vic. févr 30 (2025): e70002.
- Atkinson MA, Warady BA. Anemia in chronic kidney disease. Pediatr Nephrol [Internet]. 1 févr 33 (2018): 227-238.
- Zadrazil J, Horak P. Pathophysiology of anemia in chronic kidney diseases: A review. Biomed Pap 159 (2015): 197-202.
- Badura K, Janc J, Wasik J, et al. Anemia of Chronic Kidney Disease-A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 12 (2024): 1191.
- Hashmi MF, Shaikh H, Rout P. Anemia of Chronic Kidney Disease. In: StatPearls (2024).
- Haase VH, Tanaka T, Koury MJ. Hypoxia-inducible factor activators: a novel class of oral drugs for the treatment of anemia of chronic kidney disease. Hematol Am Soc Hematol Educ Program 2024 (2024): 409-418.
- Aseneh JB, Kemah BLA, Mabouna S, et al. Chronic kidney disease in Cameroon: a scoping review. BMC Nephrol 21 (2020): 409.
- Gbadegesin RA, Ulasi I, Ajayi S, et al. APOL1 Bi- and Monoallelic Variants and Chronic Kidney Disease in West Africans. N Engl J Med 392 (2025): 228-238.
- Njamnshi RK, Maimouna M, Ngarka L, et al. A retrospective cohort study on the cost-effectiveness analysis of kidney transplantation compared to dialysis in Cameroon: evidence for policy. Pan Afr Med J 46 (2023).
- GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol 23 (2024): 973-1003.