Evaluation of the Bioequivalence of two Linagliptin/Metformin Hydrochloride 2.5/1000 mg Fixed-Dose Combination Tablets in Healthy Adults Under Fed Conditions: A Study from Bangladesh
Sabrina Akter Tushi*, Md. Ashiqur Rahman, Uttom Kumar Bhowmik, Nayan Ghosh, Nithon Chandra Sahana, Md. Ashraful Islam
Novus Clinical Research Services Limited, Dhaka, Bangladesh
*Corresponding author: Sabrina Akter Tushi, Novus Clinical Research Services Limited, Dhaka, Bangladesh.
Received: 02 October 2025; Accepted: 08 October 2025; Published: 18 October 2025
Article Information
Citation: Sabrina Akter Tushi, Md. Ashiqur Rahman, Uttom Kumar Bhowmik, Nayan Ghosh, Nithon Chandra Sahana, Md. Ashraful Islam. Evaluation of the Bioequivalence of two Linagliptin/Metformin Hydrochloride 2.5/1000 mg Fixed-Dose Combination Tablets in Healthy Adults Under Fed Conditions: A Study from Bangladesh. Journal of Pharmacy and Pharmacology Research. 9 (2025): 135-143.
Share at FacebookAbstract
Background: Fixed-dose combinations (FDCs) of linagliptin and metformin hydrochloride are commonly used in the treatment of type 2 diabetes mellitus (T2DM) due to their complementary mechanisms of action. Establishing bioequivalence between a generic formulation and an innovator product is essential to ensure comparable safety and efficacy.
Aims: To evaluate the bioequivalence of a test formulation of linagliptin and metformin hydrochloride 2.5/1000 mg tablets with the reference product, Trajentamet®, under fed conditions in healthy adult Bangladeshi subjects.
Methods: In this randomized, open-label, two-period, two-sequence, crossover study, healthy volunteers received a single dose of the test or reference product under fed conditions, with a 7-day washout period between doses. Plasma concentrations of linagliptin and metformin were determined using validated LC-MS/MS methods. Pharmacokinetic parameters, including Cmax, AUC0-t, and AUC0-∞, were calculated. Bioequivalence was assessed using 90% confidence intervals (CIs) for the test-to-reference (T/R) geometric mean ratios, with acceptance criteria of 80%–125%. Statistical analysis was performed using SAS® software, calculating the ratios of least square means and confidence intervals for primary pharmacokinetic parameters.
Results: A total of 24 healthy male subjects (mean age 24.09 ± 3.26 years) completed the study. The T/R ratio of Least Squares Geometric Means and 90% confidence intervals for log-transformed data for Cmax and AUC measures of Linagliptin and Metformin were within the bioequivalence range of 80%–125%. For Linagliptin, Cmax was 103.55% and AUC0-72 was 103.81%. For Metformin, Cmax was 98.99%, AUC0-t was 100.45% and AUC0-∞ was 100.19%, all within the bioequivalence range of 80%–125% for log-transformed values. Statistical analysis (ANOVA) confirmed no significant differences between the formulations, supporting bioequivalence for both drugs.
Conclusion: The test formulation of linagliptin and metformin hydrochloride 2.5/1000 mg tablets is bioequivalent to Trajentamet® under fed conditions in healthy Bangladeshi adults. These findings support its use as a safe and effective alternative in the management of T2DM.
Keywords
Bioequivalence, Linagliptin, Metformin, Fixed-dose combination, Type 2 diabetes mellitus, Pharmacokinetics, Trajentamet®, Bangladesh
Bioequivalence articles, Linagliptin articles, Metformin articles, Fixed-dose combination articles, Type 2 diabetes mellitus articles, Pharmacokinetics articles, Trajentamet articles, Bangladesh articles
Article Details
Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by persistent hyperglycemia resulting from defects in insulin secretion, insulin action, or both, and is associated with long-term complications such as cardiovascular disease, nephropathy, neuropathy, and retinopathy [1]. The global burden of diabetes continues to rise, with the International Diabetes Federation estimating that approximately 9.3% of adults aged 20–79 years are currently affected [2]. Bangladesh has witnessed a particularly sharp increase in diabetes prevalence, creating significant challenges for public health systems and necessitating the availability of effective and affordable therapeutic interventions [2]. Type 2 diabetes mellitus (T2DM), the predominant form of the disease, is progressive in nature and often requires combination therapy to achieve adequate glycemic control. Combination regimens that target multiple pathophysiological pathways can enhance treatment efficacy and delay the need for insulin therapy [3]. Among such regimens, the fixed-dose combination (FDC) of linagliptin and metformin hydrochloride has gained prominence due to its complementary mechanisms of action and favorable safety profile [4].
Linagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, enhances the incretin effect by prolonging the action of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), thereby promoting glucose-dependent insulin secretion and suppressing glucagon release [5]. Its pharmacokinetic properties—particularly its predominantly non-renal elimination—render it suitable for use in patients with renal impairment without the need for dose adjustments [6]. Metformin hydrochloride, a biguanide, remains the first-line pharmacologic therapy for T2DM. It decreases hepatic glucose production and improves insulin sensitivity, contributing significantly to glycemic control and long-term cardiovascular benefits [7,8]. The FDC of linagliptin and metformin not only combines the benefits of both agents but also simplifies treatment regimens, which can lead to improved medication adherence and better clinical outcomes [4,9]. However, the high cost of branded products such as Trajentamet® can limit access, particularly in low- and middle-income countries. In this context, the availability of cost-effective generic alternatives becomes essential. Bioequivalence (BE) studies are a critical component of the regulatory approval process for generic formulations. They aim to demonstrate that the generic product has similar pharmacokinetic characteristics to the reference product under specific conditions, ensuring therapeutic equivalence without compromising safety or efficacy [10]. Although BE studies of linagliptin/metformin combinations have been conducted in various populations, data from South Asian cohorts remain limited. Moreover, fed-state BE evaluations are particularly important for metformin-containing products due to the influence of food on its absorption profile [11,12]. Given the rising burden of T2DM in Bangladesh and the need for affordable treatment options, this study was designed to evaluate the bioequivalence of a newly developed FDC of linagliptin and metformin hydrochloride 2.5/1000 mg tablets—produced by a local pharmaceutical manufacturer—compared to the reference product, Trajentamet®, under fed conditions in healthy Bangladeshi adult volunteers.
Methods and Materials
Study Design
This was an open-label, balanced, randomized, two-treatment, single-period, parallel-group, single-dose bioequivalence study conducted in healthy adult male subjects under fed conditions. The study was conducted over a 72-hour period, with a 10-hour fasting period prior to dosing. A high-fat, high-calorie meal was administered 30 minutes before drug administration, followed by 240 mL of a 20% glucose solution. Blood samples were collected pre-dose and at various time points up to 72 hours post-dose. To ensure there was no carryover effect, an adequate washout period was maintained between treatments.
Study Center and Study Period
The study was conducted at one of Bangladesh’s earliest DGDA-approved Contract Research Organizations (CRO). The clinical phase of the study took place at Novus Clinical Research Services Limited from March 9 to March 13, 2023, while the analytical stage was carried out from March 27 to April 17, 2023.
Ethical Standards/Compliance with Ethics Guidelines
This study was conducted in accordance with the approved protocol and ethical principles that have their origin in the Declaration of Helsinki and that are consistent with the current ICH- GCP. The study documents, including the protocol and consent form, were reviewed and approved by the Bangladesh Medical Research Council (BMRC) of the National Research Ethics Committee (NREC) in October 2022 (Reference No.: BMRC/NREC/2022-2025/324). The study was also approved by the Directorate General of Drug Administration (DGDA) in January 2023 (Reference No.: DGDA/CTP-04/2016/2782).
Study Products
Table 01 provides the identification details of the investigational products (IMPs) used in this study.
Table 01: Identification of the investigational product (s).
|
IMP details |
Test product (T) |
Reference product (R) |
|
Name of IMP |
Linagliptin and Metformin, 2.5/1000 mg tablet |
Trajentamet 2.5/1000 mg (Linagliptin and Metformin Hydrochloride, 2.5/1000 mg) |
|
Formulation |
Tablet |
Tablet |
|
Batch/Lot No. |
LTN (092/21) 200C |
D54909 |
|
Manufacturing Date |
Feb’ 2023 |
N/A |
|
Expiry Date |
Jan’ 2025 (Tentative) |
June’ 2024 |
|
Name and Address of the manufacturer |
Beximco Pharmaceuticals Limited, Bangladesh |
Boehringer Ingelheim pty Ltd. |
Study Subjects
The study included 24 healthy male volunteers aged 20–30 years with a BMI of 18.60–29.60 kg/m². All subjects gave written informed consent before screening and check-in. Screening involved medical history, physical exam, vital signs, ECG, chest X-ray, laboratory investigation (haematology, biochemistry, urinalysis, serology), and a urine drug abuse test. Only those with normal values were enrolled as subjects. Subjects were excluded for hypersensitivity to study drugs, abnormal vital signs, difficulty swallowing tablets, or significant medical conditions. Other exclusions included recent illness, hospitalization, blood loss (>500 mL), prior study participation (within 3 months), or use of medications, recreational drugs, alcohol, xanthine-containing foods, or grapefruit within restricted timeframes.
Standard Meal and Fluid
Standardized meal was given during check-in (in such a way to maintain at least 10.00 hours fasting prior to breakfast), high fat, high calorie breakfast at 30 minutes prior to dosing and standard meals at around 04.00, 08.00, 12.00, 25.00, 29.00, 33.00, 37.00, 49.00 hours post-dose in each study period, subjects were allowed to drink any amount of water they desired.
Blood Sampling
Blood samples were collected through a cannula at various time points (0.00, 0.33, 0.67, 1.00, 1.33, 1.67, 2.00, 2.50, 3.00, 3.50, 4.00, 4.50, 5.00, 6.00, 8.00, 10.00, 12.00, 16.00, 24.00, 36.00, 48.00, and 72.00 hours post-dose). A 0.5 mL saline solution was infused after each sample, except for pre-dose and ambulatory samples. The first 0.5 ml of each sample was discarded, except for pre-dose and ambulatory samples. Plasma was separated within 60 minutes by centrifuging the vacutainers at 3500 RPM for 10 minutes at 5°C ±3°C. Plasma aliquots (2.0 ml each) were stored in duplicate at -20°C±5°C for analysis. After collection of blood sample at each time point, sample was transferred to analytical before centrifugation and plasma is stored at analytical freezer after centrifugation.
Safety Assessment
Physical and vital examinations (blood pressure, pulse rate, respiration rate, and body temperature) were performed at screening, check-in, check-out and at 1.00, 3.00, 5.00, 7.00, 9.00, 13.00, 26.00 and 35.00 hours post dose in each study period. Additional well-being checks were done during ambulatory post-dose at each period. Laboratory investigations were conducted at the time of screening and at the end of the study to ensure safety throughout the trial.
Analytical Method
Blood samples were collected in K2EDTA tubes, and immediately after sampling, they were centrifuged at 3500 RPM for 10 minutes at 5 oC ± 3 oC. Following sample separation, the supernatants were stored below -70 oC until analyzed further. The chromatographic separation was performed Zorbax Eclipse (XDB-C18, 4.6 × 150 mm, 5.0 μm) column. In the positive electrospray mode, the mass spectrometer was used. The analytical method involved a 0.100 mL human plasma sample, with extraction performed using the protein precipitation method. Plasma samples were analyzed using a validated LC-MS/MS method, with Olmesartan as the internal standard. The linearity range for Linagliptin was 200-10,000 pg/mL, and for Metformin, it was 10-3,000 ng/mL, sufficient to quantify the expected concentration range of the drugs in plasma following the proposed dose of Linagliptin and Metformin Hydrochloride 2.5/1000 mg tablet. The method’s precision and accuracy were evaluated using quality control samples at four concentrations (Linagliptin: 600.00, 750.00, 3000.00, 7500.00 pg/mL; Metformin: 25.00, 250.00, 1000.00, 2500.00 ng/mL), which were evenly distributed among the plasma samples of participants. This validated method ensured accurate and reliable pharmacokinetic assessments of Linagliptin and Metformin in plasma samples.
Statistical Analysis
Statistical analysis was performed using SAS® software (Version 9.0). The 90% confidence intervals for the ratio of least square means (Test to Reference) and the power of the ANOVA to detect a 20% difference were calculated using LSMEAN values and standard errors. Bioequivalence was assessed based on the least square mean ratios and 90% confidence intervals for Cmax, AUC0-t, and AUC0-∞. To be considered bioequivalent, the T/R ratio and 90% CI for these parameters should fall within 80.00% to 125.00%.
Results
Demographic Details
A total of 47 volunteers were screened for the study and 24 subjects were successfully enrolled and randomized into two groups: 12 subjects in the Test (T) group and 12 subjects in the Reference (R) group. Both groups received the required doses, with 12 subjects in each group being dosed. All 24 enrolled subjects completed the study and were evaluated. No subjects were discontinued during the study. Table 02 presents the demographic characteristics of the study subjects.
Table 02: Demographic Characteristics of the Subjects (n= 24).
|
Characteristics |
Values |
|
Age (years) |
24.09 ± 3.255 years |
|
Height (cm) |
167 ± 5.31 cm |
|
Weight (kg) |
63.89 ± 9.399 kg |
|
BMI (kg/m²) |
22.895 ± 3.008 |
Pharmacokinetic and Statistical analysis
The summary of pharmacokinetic parameters estimated for both analytes and of the Reference product-R and Test product-T are summarized in table 03 (a) and 03 (b). For Linagliptin, as truncation approach was applied, only Cmax, Tmax and AUC0-72 PK Parameter were calculated.
Table 03 (a): Summary of Pharmacokinetic Parameters
|
Linagliptin (Reference Product) |
|||||||
|
Variable |
N |
Arithmetic |
SD |
CV% |
Min |
Median |
Max |
|
Mean |
|||||||
|
Tmax (hr) |
12 |
6.867 |
4.108 |
59.8 |
1.35 |
6.01 |
12 |
|
Cmax (pg/mL) |
12 |
4711.976 |
1286.678 |
27.3 |
3004.26 |
4753.7 |
6719.58 |
|
AUC0-t (hr*pg/mL) |
12 |
197628.6 |
47502.25 |
24 |
131772.9 |
192679.6 |
299074.4 |
|
Linagliptin (Test Product) |
|||||||
|
N |
Arithmetic |
SD |
CV% |
Min |
Median |
Max |
|
|
Mean |
|||||||
|
Tmax (hr) |
12 |
5.857 |
3.159 |
53.9 |
1.67 |
5.5 |
12 |
|
Cmax (pg/mL) |
12 |
4834.597 |
1157.846 |
23.9 |
3196.48 |
4855.56 |
6718.53 |
|
AUC0-t (hr*pg/mL) |
12 |
206714 |
58937.08 |
28.5 |
144121.6 |
188484.3 |
342209.7 |
Table 03 (b): Summary of Pharmacokinetic Parameters
|
Metformin (Reference Product) |
|||||||
|
Variable |
N |
Arithmetic |
SD |
CV% |
Min |
Median |
Max |
|
Mean |
|||||||
|
Tmax (hr) |
12 |
5.188 |
2.122 |
40.9 |
1.33 |
5.5 |
8.12 |
|
Cmax (ng/mL) |
12 |
1808.846 |
399.888 |
22.1 |
1307.47 |
1791.93 |
2687.51 |
|
AUC0-t (hr*ng/mL) |
12 |
19384.9 |
3632.635 |
18.7 |
12461.74 |
19074.46 |
26978.59 |
|
AUC0-∞ (hr*ng/mL) |
12 |
19556.77 |
3628.951 |
18.6 |
12672.47 |
19238.66 |
27172.86 |
|
AUC_% Extrap_obs (%) |
12 |
0.913 |
0.493 |
53.9 |
0.4 |
0.74 |
1.82 |
|
T½ (hr) |
12 |
6.593 |
2.168 |
32.9 |
4.4 |
6.12 |
12.42 |
|
Kel (hr-1) |
12 |
0.113 |
0.027 |
24.2 |
0.06 |
0.11 |
0.16 |
|
Metformin (Test Product) |
|||||||
|
N |
Arithmetic |
SD |
CV% |
Min |
Median |
Max |
|
|
Mean |
|||||||
|
Tmax (hr) |
12 |
5.042 |
1.473 |
29.2 |
1.33 |
5.99 |
6.05 |
|
Cmax (ng/mL) |
12 |
1803.876 |
439.87 |
24.4 |
1085.74 |
1720.23 |
2539.98 |
|
AUC0-t (hr*ng/mL) |
12 |
19702.58 |
4431.723 |
22.5 |
10152.12 |
19141.13 |
24434.15 |
|
AUC0-∞ (hr*ng/mL) |
12 |
19822.88 |
4425.657 |
22.3 |
10281.03 |
19236.98 |
24577.89 |
|
AUC_% Extrap_obs (%) |
12 |
0.652 |
0.29 |
44.6 |
0.33 |
0.62 |
1.25 |
|
T½ (hr) |
12 |
5.431 |
1.524 |
28.1 |
3.89 |
4.74 |
8.63 |
|
Kel (hr-1) |
12 |
0.136 |
0.032 |
23.6 |
0.08 |
0.15 |
0.18 |
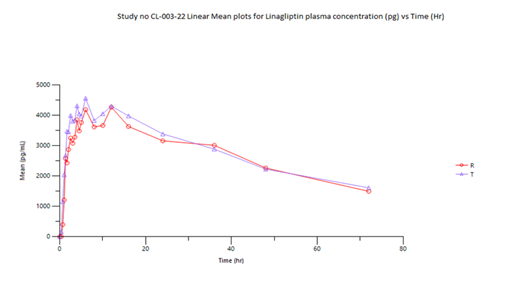
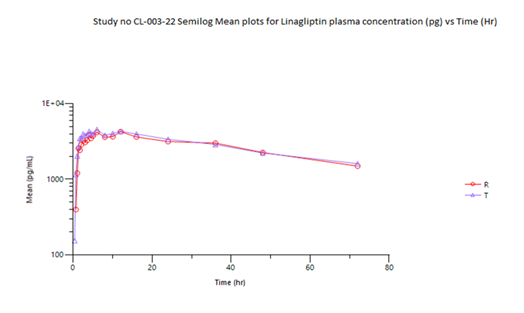
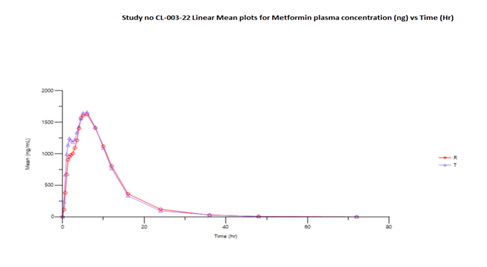
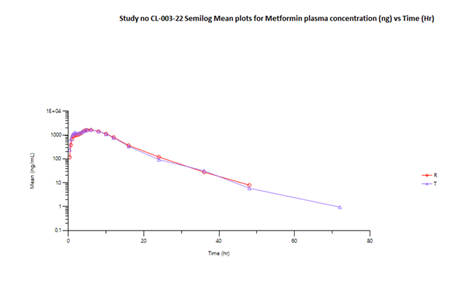
The mean Cmax obtained for Linagliptin in reference and test product was 4711.976 pg/mL and 4834.597 pg/mL respectively. The mean Cmax obtained for Metformin in reference and test product was 1808.846 ng/mL and 1803.876 ng/mL respectively. The mean area under the curve from zero to up to 72 hours concentration for Linagliptin in reference and test product was 197628.6 (hr*pg/mL) and 206714 (hr*pg/mL) respectively. The mean area under the curve from zero to last measurable concentration for Metformin in reference and test product was 19384.9 (hr*ng/mL) and 19702.58 (hr*ng/mL) respectively. The mean area under the curve from zero to infinity for Metformin in reference and test product was 19556.77 (hr*ng/mL) and 19822.88 (hr*ng/mL) respectively.
Table 04 (a) shows the bioequivalence results for Linagliptin. The Test product's Cmax (4706.567 pg/mL) and AUC0-72 (199,979.102 hr*pg/mL) were compared to the Reference product (4545.151 pg/mL and 192,644.092 hr*pg/mL, respectively). The T/R ratios were 103.55% for Cmax and 103.81% for AUC0-72, with 90% confidence intervals (86.01%-124.67%) for Cmax and 87.20%-123.57% for AUC0-72 both within predefined acceptable the bioequivalence range 80%-125%.
Table 04 (a): Summary Results (Linagliptin)
|
Parameter |
Geometric Least Squares Means (GEOLSM) |
T/R Ratio (%) |
90% Confidence Interval |
Inter Subject CV (%) |
Power (%) |
||
|
Test Product |
Reference Product |
Lower Limit (%) |
Upper Limit (%) |
||||
|
Cmax (pg/mL) |
4706.567 |
4545.151 |
103.55 |
86.01 |
124.67 |
26.94 |
79.67 |
|
AUC0-72 (hr*pg/mL) |
199979.102 |
192644.092 |
103.81 |
87.2 |
123.57 |
25.25 |
84.41 |
Table 04 (b) presents the bioequivalence results for Metformin. The Cmax for the Test product (1754.424 ng/mL) compared to the Reference product (1772.370 ng/mL) showed a T/R ratio of 98.99%, within the 90% confidence interval of 84.34%-116.18%. Similarly, the AUC0-t (19155.963 hr*ng/mL) and AUC0-∞ (19281.681 hr*ng/mL) for the Test product were compared to the Reference product (19069.617 hr*ng/mL and 19245.634 hr*ng/mL), showing T/R ratios of 100.45% and 100.19%, respectively, with 90% confidence intervals of 85.56%-117.94% and 85.47%-117.44%. This confidence interval is within the predefined bioequivalence range of 80% - 125% for the log transformed data values.
Table 04 (b): Summary Results (Metformin)
|
Parameter |
Geometric Least Squares Means (GEOLSM) |
T/R Ratio (%) |
90% Confidence Interval |
Inter Subject CV (%) |
Power (%) |
||
|
Test Product |
Reference Product |
Lower Limit (%) |
Upper Limit (%) |
||||
|
Cmax (ng/mL) |
1754.424 |
1772.37 |
98.99 |
84.34 |
116.18 |
23.14 |
89.82 |
|
AUC0-t (hr*ng/mL) |
19155.963 |
19069.617 |
100.45 |
85.56 |
117.94 |
23.19 |
89.7 |
|
AUC0-∞ (hr*ng/mL) |
19281.681 |
19245.634 |
100.19 |
85.47 |
117.44 |
22.96 |
90.24 |
The log-transformed pharmacokinetic parameters (Cmax, AUC0-t and AUC0-∞) for Metformin and Cmax and AUC0-72 for Linagliptin were analyzed using an ANOVA model. The ANOVA results for Linagliptin (Table 05a) and Metformin (Table 05b) show p-values for Cmax and AUC parameters are all above 0.05, indicating no significant differences between the Test and Reference formulations.
Table 05 (a): Analysis of Variance (ANOVA) (Linagliptin)
|
ANOVA p Values |
||
|
Parameters |
LCmax |
LAUC0-72 |
|
Formulation |
0.7498 |
0.7163 |
Table 05 (b): Analysis of Variance (ANOVA) (Metformin)
|
ANOVA p Values |
|||
|
Parameters |
LCmax |
LAUC0-t |
LAUC0-∞ |
|
Formulation |
0.9141 |
0.9619 |
0.984 |
Discussion
The pharmacokinetic evaluation of the test and reference formulations of linagliptin/metformin hydrochloride under fed conditions demonstrated highly comparable exposure profiles across all primary bioavailability parameters. For metformin, both formulations showed minimal differences in Tmax (5.042 hours for the test vs. 5.188 hours for the reference), indicating a similar onset of absorption. The Cmax values were nearly identical (1,803.9 ng/mL vs. 1,808.8 ng/mL), while the AUC0-t and AUC0-∞ ratios were 100.45% and 100.19%, respectively, well within the regulatory acceptance interval of 80–125% for bioequivalence [10,13]. The Cmax T/R ratio of 98.99% further supports equivalence in the rate and extent of absorption. For linagliptin, pharmacokinetic metrics similarly confirmed equivalence. The Cmax was marginally higher for the test product (4,834.597 pg/mL) compared with the reference (4,711.976 pg/mL), yielding a T/R ratio of 103.55%. The AUC0-t values (206,714 hr*pg/mL vs. 197,628.6 hr*pg/mL) resulted in a T/R ratio of 103.81%. All 90% confidence intervals for Cmax and AUC were within bioequivalence boundaries, consistent with earlier reports of linagliptin pharmacokinetics under both fasting and fed conditions [14–16].
Statistical analyses revealed no significant differences between formulations. For linagliptin, p-values for Cmax (0.7498) and AUC0-72 (0.7163) indicated equivalence, while for metformin, p-values for Cmax (0.9141), AUC0-t (0.9619), and AUC0-∞ (0.9840) further confirmed the absence of statistically meaningful variability. These findings align with regulatory expectations that equivalence should be demonstrated primarily through confidence interval analysis rather than hypothesis testing [10,13].
Variability in absorption windows (e.g., 1.33–8.12 hours for metformin reference vs. 1.33–6.05 hours for test; 1.35–12.0 hours for linagliptin reference vs. 1.67–12.0 hours for test) reflects expected inter-individual differences in drug absorption under fed conditions [15,17]. The close alignment of median absorption times between test and reference further underscores the similar pharmacokinetic behavior of both formulations. Overall, the study confirms that the test linagliptin/metformin hydrochloride 2.5/1000 mg fixed-dose combination tablet is bioequivalent to the reference product, Trajentamet® (Boehringer Ingelheim). This conclusion satisfies regulatory and clinical benchmarks, providing evidence for therapeutic equivalence. The availability of a bioequivalent formulation has important implications for affordability and access to combination therapy for type 2 diabetes mellitus, particularly in low- and middle-income settings such as Bangladesh.
Conclusions
This study demonstrated that the test formulation of linagliptin/metformin hydrochloride 2.5/1000 mg tablets is bioequivalent to the reference product, Trajentamet® (Boehringer Ingelheim), under fed conditions in healthy Bangladeshi adult volunteers. The 90% confidence intervals for the ratios of Cmax, AUC0-t, and AUC0-∞ for both linagliptin and metformin were contained within the regulatory acceptance range of 80%–125%, with no statistically significant differences observed between formulations. These results confirm that the test formulation fulfills established regulatory requirements for bioequivalence and can therefore be considered therapeutically interchangeable with the reference product. Importantly, the introduction of a locally manufactured, cost-effective fixed-dose combination may enhance treatment accessibility, affordability, and adherence for patients with type 2 diabetes mellitus in Bangladesh and similar low- and middle-income settings.
Declarations
Acknowledgments
The authors express their gratitude to all the volunteers who participated in the study. The authors also acknowledge the study sponsor, Beximco Pharmaceuticals Limited, for providing financial support and supplying the trial medication.
Funding
This study was sponsored by Beximco Pharmaceuticals Limited.
Conflict of Interest
The authors declare no conflicts of interest relevant to the content of this article.
Ethics Approval
This study was conducted in accordance with the approved protocol and in accordance with the ethical principles outlined in the Declaration of Helsinki and the ICH-GCP guidelines. The study documents, including the protocol and informed consent form, were reviewed and approved by the Bangladesh Medical Research Council (BMRC) and the National Research Ethics Committee (NREC) in October 2022 (Reference No.: BMRC/NREC/2022-2025/324). The study was also approved by the Directorate General of Drug Administration (DGDA), Bangladesh, in January 2023 (Reference No.: DGDA/CTP-04/2016/2782).
Consent to Participate
All participants provided written informed consent after receiving complete and pertinent information about the research.
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- González-Ortíz M, Martínez-Abundis E, Robles-Cervantes JA, et al. Effect of rosiglitazone on endothelial function in type 2 diabetic patients with coronary artery disease. Diabetes Res Clin Pract 81 (2008): 164–8.
- International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation (2019).
- Polavarapu B, Suda M, Imran M. Fixed-dose combinations in type 2 diabetes management: pharmacokinetic and pharmacodynamic considerations. Curr Clin Pharmacol 15 (2020): 118–26.
- Regazzi MB, Merico V, Molinelli A. Linagliptin/metformin hydrochloride in the treatment of type 2 diabetes: a review. Drugs Today (Barc) 50 (2014): 599–613.
- Först T, Pfützner A. Linagliptin, a novel DPP-4 inhibitor in type 2 diabetes: a systematic review of the literature. Expert Opin Investig Drugs 20 (2011): 381–9.
- Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 6 (2015): 86.
- Rojas LB, Gomes MB. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 5 (2013): 6.
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50 (2011): 81–98.
- Polavarapu B, Pathak R, Purohit AK. Linagliptin/metformin fixed-dose combination in the management of type 2 diabetes mellitus. Drugs RD 20 (2020): 237–46.
- US Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations (2003).
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. London: EMA (2010).
- Dey S. Importance of bioequivalence studies in the development of generic drugs. Int J Pharm Sci Res 8 (2017): 1447–54.
- Chow SC, Liu JP. Design and Analysis of Bioavailability and Bioequivalence Studies. 3rd ed. Chapman & Hall/CRC Biostatistics Series (2009).
- Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet 51 (2012): 411–27.
- Bergmann A, Lonnecker M, Graefe-Mody U, et al. Pharmacokinetics and bioequivalence of single-dose linagliptin in healthy subjects under fasting and fed conditions. Clin Pharmacol Drug Dev 9 (2020): 850–7.
- Xue L, Zhou Y, Fan H, et al. Bioequivalence study of two brands of linagliptin tablets in fasting and fed conditions. J Exp Res Pharm 7 (2022): 35–43.
- Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 10 (2019): 389.