Expression of Matrix Metalloproteinases -1, -2 and -9 Associated with Pelvic Organ Prolapse : Experimental Analytical Study of The Uterosacral Ligaments of Congolese Women from The City of Kananga in DR Congo
Antoine Tshimbundu Kayembe*,1, Patrick Kahindo Muyayalo2, Andy Mbangama Muela2, Rahma Raschid Tozin2
1Department of Gynaecology and Obstetrics, Faculty of Medicine, University Notre-Dame of Kasayi, Central Kasaï, D.R. Congo.
2Department of Gynaecology and Obstetrics, Faculty of Medicine, University of Kinshasa, Kinshasa, D.R. Congo
*Corresponding author: Antoine Tshimbundu Kayembe, Department of Gynaecology and Obstetrics, Faculty of Medicine, University Notre-Dame of Kasayi, Central Kasaï, D.R. Congo.
Received: 24 September 2025; Accepted: 03 October 2025; Published: 21 October 2025
Article Information
Citation: Antoine Tshimbundu Kayembe, Patrick Kahindo Muyayalo, Andy Mbangama Muela, Rahma Raschid Tozin. Expression of Matrix Metalloproteinases -1, -2 and -9 Associated with Pelvic Organ Prolapse : Experimental Analytical Study of The Uterosacral Ligaments of Congolese Women from The City of Kananga in DR Congo. Obstetrics and Gynecology Research. 8 (2025): 177-185.
Share at FacebookAbstract
Objective : The objective of this study is to determine the level of expression of MMP-1, MMP-2 and -9 in prolapsed tissues of the uterosacral ligament and identify the types of MMP most associated with pelvic organ prolapse during the mass campaign in the city of Kananga in the Democratic Republic of Congo.
Methods : This is an experimental analytical study based on immunohistochemical examination of the uterosacral ligaments of 100 consenting patients divided into 2 groups and admitted to the gynecology departments of Bon-Berger hospitals in Tshikaji and Saint-Georges in Katoka, from January 1 to July 31, 2023. Case selection is based on non-probability convenience sampling. The Anova test, the Chi-square test and logistic regression with adjustment are used in statistical analyses.
Results: The expression of MMP-1, -2 and -9 in the uterosacral ligaments is significantly increased in the study group with a mean immunoreactivity of 40.38±19.10%(P=0.000) for MMP-1, 35.20±20.50%(P=0.000) for MMP-2 and 45.94±17.80%(P=0.000) for MMP-9. Positive immunoreactivities to matrix metalloproteinases-1, -2 and -9 are significantly associated with the occurrence of pelvic organ prolapse. Positive immunoreactivity to matrix metalloproteinase-1 (aOR: 6.36, 95%CI:1.167-34.64, P: 0.033) and matrix metalloproteinase-9 (aOR: 5.37, 95%CI:1.519-23.786, p: 0.011) are more determining in the occurrence of pelvic organ prolapse in the city of Kananga.
Conclusion: The expression of matrix metalloproteinase-1, - and -9 is increased in prolapsed uterosacral ligaments and associated with this condition and matrix metalloproteinase-1 and 9 are determining in the occurrence of pelvic organ prolapse in our city of Kananga.
Keywords
Matrix metalloproteinases-1, Matrix metalloproteinases-2, Matrix metalloproteinases-9, Uterosacral ligaments, Pelvic organ prolapse, Kananga
Article Details
Introduction
Pelvic organ prolapse (POP) is a multifactorial disease associated with major morbidity in women in both high- and low-income countries and has many predisposing factors known worldwide such as vaginal delivery, advanced age, obesity, menopause, constipation, racial and genetic factors and connective tissue diseases [1,2,3,4]. WHO estimates that POP affects nearly 50% of women over 50 years of age and its lifetime prevalence ranges from 20 to 50%. Which is one of the most common causes of gynecologic surgery [5,6,7,8]. The lifetime risk for a woman to undergo surgery for pelvic organ prolapse is estimated at 11.1%, with a 10-year re-intervention rate of 17% [5,9,10]. The global prevalence of POP has been reported to be approximately 9.00% and it is however estimated that this figure is closer to 20.00% in low-income countries [11]. In Africa, several studies conducted in sub-Saharan countries such as Gambia [12], Ghana [13], Ethiopia [14], and Tanzania [15] have reported prevalences ranging from 12 to 65.00%. This prevalence is 24.12% in our city of Kananga in the Democratic Republic of Congo [16].
POP is a pathology of pelvic connective tissues characterized by their reduced collagen content linked either to a defect in its production or to its massive degradation [1,17,18,19,20,21,22]. Several studies report that the reduced collagen content leads to a significant decrease in the tensile strength of ligament tissues [18,23,24,25]. The massive degradation of collagen is ensured by secreted proteases, the most important of which are matrix metalloproteinases (MMPs) [1,17,18,19,20,21,22]. These MMPs are under the control of tissue inhibitors (TIMPS) [1,25,26,27], growth factors such as transforming growth factor β (TGF-β) [28,29,30,31], platelet derived growth factor (PDGF) [27]; inflammatory mediators such as interleukins [27], tumor necrosis factor-α (TNF-α) [31]; and hormones such as estrogens, progesterone and corticosteroids [19,23,26,32]. A study conducted in our city of Kananga reported the factors associated with POP following intense physical work, malnutrition in the form of BMI less than 18.5 kg/m2, multiparity, vaginal delivery, fetal macrosomia, obstetric trauma and menopause [33] while the Kinshasa study had identified multiparity, obesity, vaginal delivery, the number of vaginal deliveries greater than 4, fetal macrosomia, obstetric trauma, menopause as the factors associated with POP [34]. Several studies demonstrate that these factors determine the increase in MMP-1, -2 and -9 at the pelvic level before the occurrence of POP [1,35,36,37,38]. The lack of data on MMPs associated with POP in our city of Kananga justifies the present study whose objective is to determine the level of expression of MMP-1, MMP-2 and -9 in the prolapsed tissues of the uterosacral ligament and to identify the types of MMPs associated with pelvic organ prolapse during the mass campaign in the two hospitals of Bon-Berger and Saint-Georges in the city of Kananga in the Democratic Republic of Congo
Methods
Study design and setting
This is an experimental analytical study comparing the study group consisting of patients suffering from pelvic organ prolapse to the comparison group consisting of patients suffering from other gynecological pathologies recorded during the mass campaign organized in the gynecological departments of two General reference hospitals in the city of Kananga: Bon-Berger Hospitals in Tshikaji and Saint Georges in Katoka, from January 1 to July 31, 2023. These two hospitals were chosen because of the presence of trained and experienced medical staff, the high attendance of patients suffering from genital prolapse, the more or less free management of genital prolapse through the various mass campaigns in the fistula cure account. Therefore, these 2 hospitals constitute references for the management of genital prolapse in the city of Kananga.
Study population
The population of our study consists of patients who have signed the informed consent form, aged between 40 and 79 years, who suffer from genital prolapse for the study group and other benign gynecological pathologies for the comparison group and who were treated during the mass campaign in the gynecology departments of the Bon-berger hospitals in Tshikaji and Saint Georges de Katoka in the city of Kananga from January 1 to July 31, 2023, and matched according to age plus or minus 3 years. Our sampling is non-probabilistic by convenience. The sample size was determined by the limitation of our study in time and space. The following criteria allowed us to include patients in the study: Patients who signed the informed consent form, aged between 40 and 79 years who suffer from genital prolapse (study group) and other gynecological pathologies (comparison group) and who underwent total hysterectomy during the mass campaign in the gynecology departments of the Bon-berger hospitals in Tshikaji and Saint Georges de Katoka in the city of Kananga from January 1 to July 31, 2023. We excluded all patients who refused to sign the informed consent form, those who had already undergone surgery for genital prolapse, those suffering from malignant gynecological pathologies and those on hormone replacement therapy in case of complicated menopause. Our sample size was calculated using the following formula:
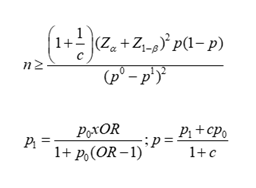
n = number of cases
c = number of cases in the comparison group
p0 = expected proportion of exposed cases in the comparison group (0.0116)
p1 = expected proportion of exposed cases in the study group (0.105)
p = proportion of exposed subjects in both groups (study and comparison) (0.058)
= Z value for the first type risk (1.645)
α = the risk of type I error (0.05)
= Z value corresponding to a surface area equal to the power of the test (1 – β). The latter constitutes the probability of finding a significant difference (1.282)
(1-β) = the desired power (0.9)
OR = minimum OR that is set for the study to be of public health interest and estimated at 20 on the basis of studies on the Visco and Yuan model [39]. The calculated sample size is greater than 47 cases and we increased it to 50 cases for the study group and 50 cases for the comparison group.
The number of cases in the comparison group is equal to the product n x c. A comparison case will be matched to a study case (c = 1) and the matching criterion is the age of patients plus or minus 3 years.
Data collection
The data were collected from the patient interview and the search of the patients' medical records and the registers of the gynecology departments of two hospitals, and recorded in the data collection record. The variables of our study are: the age of the patients, diagnosis, types of tissues collected, expression level of MMP-1, that of MMP-2, and that of MMP-9. Our data were collected as follows: after written and signed consent for each patient admitted to the study, information collection will be done by interviewing patients and searching medical records. Tissue biopsies of 10mm length of the uterosacral ligaments are taken from each woman during surgery. These biopsies were fixed in 10% formalin and preserved until the time of immunohistochemical analysis.
Immunohistochemical staining
All tissue samples were systematically fixed in 10% buffered formalin and transformed into paraffin blocks by routine methods. Sections of the samples at 5 µm thickness were made using a microtome, mounted on coated slides and stained using the double immunolabeling technique whose protocol is established in the anatomopathological laboratory of the University Clinics of Kinshasa. Each sample was stained with hematoxylin and eosin (H&E) and immunohistochemical stains using antibodies against MMP-1, MMP-2, and MMP-9.
- The staining procedure was as follows:
After fixing the samples in 10% formalin and paraffin, the samples were sectioned at 5 µm thickness using a microtome. The paraffin sections mounted on the microscope slide were first incubated overnight at 60 °C, then deparaffinized, and finally hydrated in descending alcohol series; they were incubated in citrate at 96-97°C for 60 minutes, peroxidase activity was blocked by incubating the slide sections in 3% hydrogen peroxide (3% H2O2, 25ml Universal DAB inhibitor, SN: 1950274, Ventana laboratories, Arizona, USA) for 15 minutes, we then incubated the slide sections in CuSO4 (Concentration: 5g/l, View universal DAB Copper, SN: 2002356, Ventana laboratories, Arizona, USA) for 15 minutes, the sections were incubated in protein block for 15 minutes (3% bovine serum albumin in phosphate buffered saline =PBS) to block nonspecific binding or minimize nonspecific reactivity, we incubated the sections overnight at 4°C in a humidified chamber with the primary monoclonal antibodies against Human MMPs due to 3 slides per sample including one slide for MMP-1 antibody (concentration: 500μg/ml, 25% agarose, anti-human MMP-1 monoclonal antibody, Sc-21731AC, Santa Cruz Biotechnology laboratories, Dallas, USA, dilution: 1:100), one for MMP-2 antibody (concentration: 500μg/ml, 25% agarose, anti-human MMP-2 monoclonal antibody, Sc-13595AC, Santa Cruz Biotechnology laboratories, Dallas, USA, dilution 1:100) and one for MMP-9 antibody (concentration: 500μg/ml, anti-human MMP-9 monoclonal antibody, SC-12759AC, Santa Cruz Biotechnology laboratories, Dallas, USA, dilution 1:100). These monoclonal antibodies were diluted in Dako Antibody Diluent (Glostrup, Denmark). We incubated these slides in the primary antibody amplifier mixed with hydrogen peroxide (concentration: 0.04% H2O2, 25ml, Universal diaminobenzidine H2O2, SN: 1895381, Ventana laboratories, Arizona, USA) for 20 minutes, we incubated the slides with the secondary antibodies (Universal HRP Multimer, 55 microgram of non-specific mouse immunoglobulin G per ml, SN: 1939485, Ventana laboratories, Arizona, USA) for 1 hour. The slides were carefully washed with distilled water and then phosphate-buffered saline (PBS) after each step of the procedure. Finally, we incubated the slides with the chromogen which is a substrate of diaminobenzidine peroxidase (Universal DAB Chromogen 2%, SN:20160923, Ventana laboratories, Arizona, USA) for 30 minutes, the sections were counterstained with hematoxylin for 5 minutes at room temperature, followed by rinsing with water and incubation in lithium carbonate to blue the hematoxylin. The sections were covered with Canada balsam and coverslip with bubble removal and drying for 1 hour, and mounted on the microscope (Olympus CX31) for examination at x400 magnification.
To control the secondary antibody, sections were produced by omitting the primary antibody and showed no staining while the positive control slides were stained with non-specific mouse immunoglobulin G and there was no background reaction.
Positive control : Positive control slides for MMP-1, MMP-2, and MMP-9 were prepared from placental tissue. In the positive control tissue, the monoclonal antibody was stained similarly. Negative control : The negative control slide was prepared from the same tissue block as the sample. As negative controls, we performed the same procedure with omission of primary antibodies (replaced by phosphate-buffered saline).
- Immunohistochemical and morphological analysis
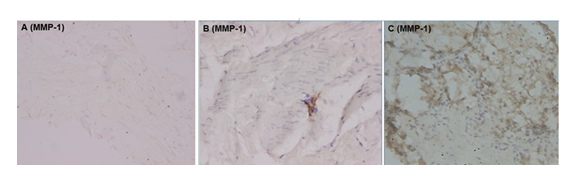
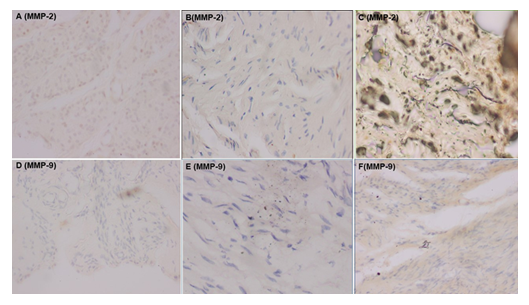
All slides were evaluated by an experienced pathologist to whom we concealed the information about the origin of the samples (from a study or comparison patient). The percentage of immunoreactive fibroblasts for MMP-1, MMP-2, and MMP-9 is recorded. In addition, the degree of immunoreactivity of the extracellular stroma is graded from 0 to 4, as follows : grade 0 corresponds to negative immunoreactivity (0%), grade 1 corresponds to 1 to 25% or focal immunoreactivity, grade 2 corresponds to 26 to 50% or focal to moderate immunoreactivity, grade 3 corresponds to 51 to 75% or moderate to diffuse immunoreactivity and grade 4 corresponds to 76 to 100% or diffuse immunoreactivity (Figures 1 and 2). Morphological aspects such as the presence of fibrosis, congestion and inflammatory infiltrate made of lymphocytes, plasma cells and macrophages are also recorded.
Statistical analyses
Data were analyzed using Statistical Package for Social Sciences (SPSS) software version 29. The ANOVA test was used to perform the intergroup comparison of means, the Chi2 test to perform the intergroup comparison of proportions, the univariate logistic regression to evaluate the strength of association between the expression (positive immunoreactivity) of MMP-1, -2 and -9 and the occurrence of genital prolapse and the multivariate logistic regression to identify the types of MMPs most determining genital prolapse. The threshold of statistical significance of our results is set at the value of p < 0.05. We adjusted the OR according to the age of the patients. Variables that were statistically significantly associated with the occurrence of Pelvic Organ Prolapse in the ANOVA and Chi-2 tests were included in the univariate analyses and those that were significantly associated with the occurrence of Pelvic Organ Prolapse in the univariate model were included in the multivariate analyses.
Ethical Considerations
Principles of medical ethics and documentary studies rules were respected : data were collected confidentially and treated anonymously. Our study obtained authorization from the Ethics Committee of the Kinshasa Health School and the local committee of different hospitals. The reference number of the approval by Ethics Committee is N°ESP/CE/19/2023. Each patient signed a preoperative informed consent form authorizing or permitting the use of tissues removed during surgery for research purposes.
Results
The mean age of patients was 57.18±8.17 years in the study group versus 56.48±8.29 years in the comparison group and there were no statistically significant differences in the mean ages between the two groups. No statistically significant differences in the presence of fibrosis, congestion and inflammatory infiltrate composed of lymphocytes, macrophages and plasma cells were found between the study and comparison groups. The expression level of MMP-1, -2 and -9 in the uterosacral ligaments was statistically significantly increased in the study group compared to the comparison group with a mean immunoreactivity of 40.38±19.10% versus 20.16±17.55% (P=0.000) for MMP-1, 35.20±20.50% versus 24.06±16.69% (P=0.000) for MMP-2 and 45.94±17.80% versus 25.42±15.50% (P=0.000) for MMP-9. In addition, positive immunoreactivities were significant in the study group compared to the comparison group for the three MMPs studied: MMP-1, MMP-2 and MMP-9 (Table 1). Univariate analyses helped us to note a significant association between the occurrence of pelvic organ prolapse and positive immunoreactivities to MMP-1, MMP-2 and MMP-9 in the uterosacral ligaments while multivariate analyses allowed us to identify positive immunoreactivities to MMP-1 (aOR: 6.36, 95%CI: 1.167-34.64, P: 0.033) and MMP-9 (aOR: 5.37, 95%CI: 1.519-26.786, P: 0.011) as the types of positive immunoreactivities in the uterosacral ligaments most associated with the occurrence of pelvic organ prolapse in the city of Kananga (Table 2).
Table 1: Immunoreactivity to MMP-1,-2 et -9 in uterosacral ligament
|
Types of MMPs |
Variables |
Study group |
Comparison group |
Total |
p |
|||
|
MMP-1 |
Mean±SD |
40.38± 19.10 |
20.16±17.55 |
30.27±18.41 |
0 |
|||
|
Grade 1 and 2 |
42 |
84% |
25 |
50% |
67 |
67% |
0.001 |
|
|
Positive Immunoreactivity |
48 |
96% |
27 |
54% |
75 |
75% |
0 |
|
|
MMP-2 |
Mean±SD |
35.20±20.50 |
24.06±16.69 |
29.63±23.84 |
0 |
|||
|
Grade 1 and 2 |
36 |
72% |
25 |
50% |
61 |
61% |
0.001 |
|
|
Positive Immunoreactivity |
46 |
92.00% |
29 |
58% |
75 |
75% |
0 |
|
|
MMP-9 |
Mean±SD |
45.94±17.80 |
25.42±15.50 |
35.68±16.65 |
0 |
|||
|
41 |
82% |
20 |
40% |
61 |
61% |
0 |
||
|
Positive Immunoreactivity |
47 |
94.00% |
24 |
48% |
71 |
71% |
0 |
|
MMP: matrix metalloproteinases; SD: Standard deviation; %: percentage; ±: more or less
Table 2: Univariable and multivariable analysis
|
Uterosacral ligaments |
||||||
|
risk factors |
Univariable analysis |
Multivariable analysis |
||||
|
aOR |
95% CI |
P-value |
aOR |
95% CI |
P-value |
|
|
Positive immunoreactivity to MMP-1 |
20.4 |
5.47-93.46 |
0.001 |
6.36 |
1.167-34.64 |
0.033 |
|
Grade 1 and 2 of MMP-1 |
1.85 |
1.03-5.60 |
0.002 |
0.89 |
0.241-1.64 |
0.36 |
|
Positive immunoreactivity to MMP-2 |
10.33 |
4.59-28.72 |
0.001 |
3.308 |
0.555-9.601 |
0.25 |
|
Grade 1 and 2 of MMP-2 |
4.4 |
1.981-9.794 |
0.003 |
0.91 |
0.21-3.13 |
0.57 |
|
Positive immunoreactivity to MMP-9 |
13.97 |
3.66-51.79 |
0.001 |
5.37 |
1.519-26.786 |
0.011 |
|
Grade 1 and 2 of MMP-9 |
8.69 |
3.260-11.84 |
0.001 |
0.54 |
0.198-9.794 |
0.342 |
aOR: ajusted odd-ratio
95% CI: 95% Confidence interval
P-value: the significant p-value set to less than 0.05
Discussion
The objectives of this study were to determine the expression level of MMPs types 1, 2 and 9 in prolapsed uterosacral ligament tissues and to identify the types of MMPs most associated with POP during the mass campaign in the two hospitals of Bon-Berger and Saint-Georges in the city of Kananga in the Democratic Republic of Congo. This study noted that the expression levels of MMP-1, -2 and -9 are significantly increased in prolapsed uterosacral ligament tissues and positive immunoreactivity to MMP-1 and -9 are more associated with pelvic organ prolapse. POP is a pathology of ligamentous connective tissues and MMPs are the main actors in the degradation of connective tissues involved in tissue remodeling [19]. The expression levels of MMP-1, MMP-2 and MMP-9 are significantly increased in the study group compared to the comparison group in our setting. This demonstrates a strong collagenolytic and gelatinolytic enzymatic activity of these MMPs in the specific degradation of collagen significantly in women suffering from pelvic organ prolapse in our setting. Our results partly meet those of Liang et al. [19], Dviri et al. [1], Usta et al. [40], Yucel et al. [41] and Vulic M et al. [42] who respectively found a significantly increased expression of MMP-2 and MMP-9, MMP-1 and MMP-9 in the prolapsed uterosacral ligaments, MMP-1 in the prolapsed uterosacral and round ligaments and MMP-1 in the uterosacral ligaments. Unlike all these cited authors, we studied at the same time the expression levels of 3 MMPs (MMP-1, MMP-2, MMP-9) which are significantly increased in the prolapsed uterosacral ligaments. On the other hand, other authors have found contradictory results, notably Strinic et al. in Croatia [17] who reported a significant increase in the expression of MMP-1 in the uterosacral ligament of women with pelvic organ prolapse but with that of MMP-2 not statistically significant while Boris et al. [21] who found a significantly increased level of MMP-2 expression with that of MMP-1 not statistically significant in the same uterosacral ligament of women suffering from pelvic organ prolapse. Which is not the case for our results. These results demonstrate the specific differences specific to each environment and each race (black for us), and can be explained by the fact that these 3 MMPs (MMP-1, MMP-2 and MMP-9) play an essential role in the degradation of collagens whose content is reduced in pelvic organ prolapse with different specificities [23,43]. Other authors have studied the level of MMP expression in the vaginal wall of women with pelvic organ prolapse and reported significantly elevated levels of either MMP-2 and MMP-9 [37,44], or MMP-9 but not MMP-2 [18,20,45], or MMP-1 [25], or MMP-1 and MMP-8 [46], or MMP-1 and MMP-3 [47,48], or MMP-1, MMP-3 and MMP-9 [49], or MMP-10 [50]. These apparently contradictory results to ours may also reflect the specific differences in the biopsy sampling sites. On the other hand, Wang et al in China reported significantly increased levels of MMP-1, MMP-2, MMP-3 and MMP-9 in the vaginal wall of women with pelvic organ prolapse [36]. This is superimposable to our results although we did not also measure MMP-3. These results demonstrate the combination of actions of different types of MMPs associated with pelvic organ prolapse in different types of pelvic connective tissues studied.
Positive immunoreactivities to MMPs are a signal of their synthesis, availability and intense enzymatic activity in the affected tissues [19,38,46]. Positive immunoreactivity to MMP-1 in the uterosacral ligaments is associated with the occurrence of POP and significantly increases the risk of prolapse by 20 in our setting. The occurrence of pelvic organ prolapse in cases of positive immunoreactivity to MMP-1 is explained by the fact that MMP-1, also called "collagenase 1", degrades type I collagen into gelatin, which is the basis of the decrease in collagen content in the pelvic ligaments, including the uterosacral ligaments, which is the source of the decrease in ligament tensile strength that determines pelvic organ prolapse [1,17,18,25,40,41]. Positive MMP-2 immunoreactivity in the uterosacral ligaments is also associated with the occurrence of pelvic organ prolapse and significantly increases the risk of prolapse by 10 in our setting. The occurrence of pelvic organ prolapse in cases of positive MMP-2 immunoreactivity is explained by the degradation, by this MMP-2 (=gelatinase A), of type I collagen into gelatin and of gelatin into simple peptides recyclable by the body. Which leads to a decrease in collagen content at the ligament level, which is the basis of a decrease in ligament tensile strength and prolapse [19,20,21,44]. Positive immunoreactivity to MMP-9 in the uterosacral ligaments is finally associated with the occurrence of pelvic organ prolapse and multiplies the risk of pelvic organ prolapse by 14 in the city of Kananga. The occurrence of pelvic organ prolapse in the case of positive immunoreactivity to MMP-9 is explained by the fact that MMP-9, otherwise known as "gelatinase B", also has both a collagenolytic activity allowing it to degrade fibrillar collagen into gelatin and a gelatinolytic activity at the basis of the degradation of gelatin into simple peptides that can be recycled by the body. This leads to a decrease in collagen content in the uterosacral ligaments, which is the basis of a decrease in ligament traction resistance and the occurrence of pelvic organ prolapse [18,19,20,37,44,45].
Even though the 3 MMPs mentioned above would be significantly associated with the occurrence of prolapse, positive immunoreactivity to MMP-1 and MMP-9 are the most determining types or most associated with the occurrence of pelvic organ prolapse in the city of Kananga. The expression of all these MMPs is inhibited by estrogens, progestins and tissue inhibitors of MMPs (TIMPs) whose intra-tissue level is low in pelvic organ prolapse [18,19,23,38,46], and activated through crossing by MMP-3, MMP-7, MMP-10, inflammatory cytokines (interleukins, TNF-α) and certain growth factors whose tissue level is high in women suffering from pelvic organ prolapse [35,37,38,46,50]. They are finally stimulated by many factors associated with pelvic organ prolapse including obstetric trauma, menopause, etc. [18,19,23,26,32]. Our results can serve as a basis for research studies on factors associated with increased expression of MMP-1, MMP-2 and MMP-9 in women suffering from POP and for experimental research studies on the inhibitory role of estrogens and progesterone on MMP-1, MMP-2 and MMP-9 in our environment (given that this inhibitory role has been proven elsewhere) with a view to adding these hormones as a means of preventing pelvic organ prolapse in women at risk in our city of Kananga as is practiced elsewhere [19,32,38,46].
The weaknesses of this study are that it did not study the expression of MMP-1, MMP-2 and MMP-9 genes in women with pelvic organ prolapse, and the levels of MMP-8, MMP-3, MMP-7 and MMP-10 associated with prolapse despite their secondary role. The strengths of our study are that it is the first to determine the level of MMP-1, MMP-2 and MMP-9 in hospital settings in the city of Kananga, the Democratic Republic of Congo and Africa; and that it is the basis for further studies to investigate the inhibitory role of estrogen-progestins and the factors associated with the high level of MMP-1, MMP-2 and MMP-9 in our setting.
Conclusion
The expression of MMP-1, MMP-2 and MMP-9 are significantly increased in the uterosacral ligament tissue of women with pelvic organ prolapse. Positive immunoreactivities to MMP-1, MMP-2 and MMP-9 are significantly associated with pelvic organ prolapse and MMP-1 and MMP-9 are the most determinant types in the environment. Our results can serve as a basis on the one hand for research studies of factors associated with the increase in the expression of MMP-1, MMP-2 and MMP-9 in women suffering from pelvic organ prolapse and on the other hand for experimental research studies of the inhibitory role of estrogens and progesterone on MMP-1, MMP-2 and MMP-9 in our environment in order to add these hormones as a means of preventing pelvic organ prolapse in women at risk in our city of Kananga.
Authors contributions
Antoine Tshimbundu Kayembe: Conception and study design, data collection, data analysis and interpretation and manuscript revision and guarantor of the study
Patrick Kahindo Muyayalo: Conception and study design, Data analysis and interpretation
Andy Mbangama Muela: Conception and study design, data analysis and interpretation
Rahma Raschid Tozin : Conception and study design, data analysis and interpretation
All authors approved final version of the manuscript: Yes
Acknowledments
To medical staff of Bon-Berger and Saint Georges Hospital (Dr Mulalu and Dr Mayi) for having allowed us and facilitated us in collecting data for this study.
To medical staff of anatomo-pathological Department of university hospital of Kinshasa (Pr Dr Lebwaze et al.) for having allowed us and facilitated us the anatomo-pathological exam of our samples
No grant was received.
Competing interests : We declare that we have no interest to disclose
References
- Dviri M, Leron E, Dreiher J, et al. Increased matrix metalloproteinases-1, -9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 156 (2011): 113–7.
- Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet 369 (2007): 1027–38.
- Nikolova G, Lee H, Berkovitz S, et al. Sequence variant in the laminin gamma1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet 120 (2007): 847–56.
- Twiss C, Triaca V, Rodríguez LV. Familial transmission of urogenital prolapse and incontinence. Curr Opin Obstet Gynecol 19 (2007): 464–8. DOI: 10.1097/GCO.0b013e3282efdc21
- Feng Y, Wang Y, Yan Bah, Lee L, Deng Y. Matrix metalloproteinase-1 expression in women with and without pelvic organ prolapse: a systematic review and meta-analysis. Clin Transl Sci 9 (2016): 267–73.
- Weber A, Holly-Eberhard R. Pelvic organ prolapse. Obstet Gynecol 106 (2005): 615–34.
- Buller J, Thompson J, Cundiff G, Krueger S, Schön Y, Courbé A. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 97 (2001): 873–9.
- Subak L, Waetjen E, Van den Eaden S, Thom D, Vittinghoff E, Brown J. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 98 (2001): 646–51.
- Olsen A, Smith V, Bergstrom J, Colling J, Clark A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89 (1997): 501–6.
- Denman M, Wong G, Boyles S, Smith V, Edwards S, Clark A. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol 198 (2008): 555–61.
- Walker G, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J 22 (2011): 127–35.
- Scherf C, Morison L, Fiander A, Ekpo G, Walraven G. Epidemiology of pelvic organ prolapse in rural Gambia, West Africa. BJOG 109 (2002): 431–6.
- Wusu-Ansah O, Opare-Addo H. Pelvic organ prolapse in rural Ghana. Int J Gynaecol Obstet 103 (2008): 121–4.
- Megabiaw B, Mulatu A, Rortveit G, Degu G, Muleta M, Blystad A, et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J 24 (2013): 1135–43.
- Masenga G, Shayo B, Rasch V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania: a population-based study in a Tanzanian rural community. PLoS One 13 (2018): 1–13.
- Tshimbundu KA, Mayi I, Mundende M, Mbangama MA, Rahma RT. Pelvic organ prolapse: a cross-sectional study during mass campaign in two hospitals in the city of Kananga in the Democratic Republic of Congo. Pan Afr Med J 47 (2024): 52.
- Strinic T, Vulic M, Tomic S, Capkun V, Stipic I, Alujevic I. Matrix metalloproteinases-1, -2 expression in uterosacral ligaments from women with pelvic organ prolapse. Maturitas 64 (2009): 132–5.
- Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol 106 (2005): 953–63.
- Liang C, Huang H, Tseng L, Chang S, Lo T, Lee C. Expression of matrix metalloproteinase-2 and tissue inhibitors of metalloproteinase-1 (TIMP-1, TIMP-2, TIMP-3) in women with uterine prolapse but without urinary incontinence. Eur J Obstet Gynecol Reprod Biol 153 (2010): 94–8.
- Phillips CH, Anthony F, Benyon C, Monga AK. Collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. Br J Obstet Gynaecol 113 (2006): 39–46.
- Boris G, Watermann D, Hancke K, Gitsch G, Werner M, Tempfer C, et al. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J 17 (2006): 478–82.
- Ewies AA, Al-Azzawi F, Thompson J. Changes in extracellular matrix proteins in the cardinal ligaments of postmenopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod 18 (2003): 2189–95.
- Kieserman-Shmokler C, Swenson C, Chen L, Desmond L, Ashton-Miller J, DeLancey J. From molecular to macro: the key role of the apical ligaments in uterovaginal support. Am J Obstet Gynecol 223 (2020): 427–36.
- Boris G, Denschlag D, Göbel H. Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J 16 (2005): 475–9.
- Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J 13 (2002): 80–7.
- Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells 9 (2020): 1076.
- De Almeida L, Hayley T, Eslambolchi Y, Chopra S, Young D, Sean G, et al. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol Rev 74 (2022): 714–70.
- Carlina GL, Bodner K, Kimberger O, Haslinger C, Schneeberger C, Horvat R, et al. The role of transforming growth factor-β (TGF-β1) in postmenopausal women with pelvic organ prolapse: an immunohistochemical study. Eur J Obstet Gynecol Reprod Biol X 7 (2020): 100111.
- Qi X, Hong L, Guo F, Fu Q, Chen L, Li B. Expression of transforming growth factor-beta1 and connective tissue growth factor in women with pelvic organ prolapse. Saudi Med J 32 (2011): 474–8.
- Li BS, Hong L, Min J, Wu D, Hu M, Guo WJ. Expression of glutathione peroxidase-1 and the collagen regulation pathway transforming growth factor-beta1–connective tissue growth factor in uterine prolapse. Exp Obstet Gynecol 40 (2013): 586–90.
- Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-beta and tumor necrosis factor-alpha–mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem 276 (2004): 22341–50.
- Moalli PA, Klingensmith WL, Meyn LA, Zyczynski HM. Regulation of matrix metalloproteinase expression by estrogen in fibroblasts derived from the pelvic floor. Am J Obstet Gynecol 187 (2002): 72–9.
- Tshimbundu KA, Kahindo MP, Mbangama MA, Rahma RT. Factors associated with pelvic organ prolapse: case-control study in two hospitals of Bon-Berger and Saint Georges in Kananga, DRC. Pan Afr Med J 48 (2024): 76.
- Tshimbundu KA, Kitenge KK, Kamba B, Tozin RR. Factors associated with genital prolapse at Saint-Joseph Hospital of Kinshasa. Pan Afr Med J 40 (2021): 234.
- Wu X, Liu X, Li T. Potential molecular targets for intervention in pelvic organ prolapse. Front Med 10 (2023): 1158907.
- Wang X, Li Y, Chen J, Guo X, Guan H, Li C. Differential expression profiling of matrix metalloproteinases and tissue inhibitors in females with or without pelvic organ prolapse. Mol Med Rep 10 (2014): 2004–8.
- Ying W, Hu Y, Zhu H. Expression of CD44, transforming growth factor-β, and matrix metalloproteinases in women with pelvic organ prolapse. Front Surg 9 (2022): 902871.
- Lei L, Ma Y, Yang H, Sun Z, Chen J, Zhu L. Polymorphisms of extracellular matrix–remodeling genes are associated with pelvic organ prolapse. Int Urogynecol J 33 (2022): 267–74.
- Visco A, Yuan L. Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol 189 (2003): 102–12.
- Usta A, Guzin K, Kanter M, Ozgül M, Usta CS. Expression of matrix metalloproteinase-1 in round ligament and uterosacral ligament tissue from women with pelvic organ prolapse. J Mol Histol 45 (2014): 275–81.
- Yucel N, Usta A, Guzin K, Kanter M, Ergun B, Ozbay N, et al. Immunohistochemical analysis of connective tissue in patients with pelvic organ prolapse. J Mol Histol 44 (2013): 97–102.
- Vulic M, Strinic T, Tomic S, Capkun V, Alujevic-Jakus I, Ivica S. Difference in expression of collagen type I and matrix metalloproteinase-1 in uterosacral ligaments of women with and without pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol 156 (2011): 83–7.
- Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse: a review. Int Urogynecol J 20 (2009): 461–74.
- Alperin M, Moalli PA. Remodeling of vaginal connective tissue in patients with prolapse. Curr Opin Obstet Gynecol 18 (2006): 544–50.
- Jackson S, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet 347 (1996): 1658–61.
- Yan H, Wu R, Li H, Gu Y, Wei W. Expression and significance of metalloproteinase and collagen in vaginal wall tissues of patients with pelvic organ prolapse. Ann Clin Lab Sci 47 (2017): 698–705.
- Skorupski P, Jankiewicz K, Miotla P, Marczak M, Kulik-Rechberger B, Rechberger T. Polymorphisms of the MMP-1 and MMP-3 genes and the risk of pelvic organ prolapse. Int Urogynecol J 24 (2013): 1033–8.
- Skorupski P, Miotla P, Jankiewicz K, Rechberger T. MMP-1 and MMP-3 gene polymorphism and the risk of pelvic organ prolapse and stress urinary incontinence. Ginekol Pol 81 (2010): 594–9.
- Ferrari MM, Rossi G, Biondi ML, Vigano P, Dell'Utri C, Meschia M. Type I collagen and matrix metalloproteinase 1, 3, and 9 gene polymorphisms in the predisposition to pelvic organ prolapse. Arch Gynecol Obstet 285 (2012): 1581–6.
- Wang H, Zhang ZQ, Wang SZ. Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse. J Minim Invasive Gynecol 22 (2015): S68.