Generation of Protective Antibodies in Mice via Inactivated Whole-Cell Vaccine Targeting Multidrug-Resistant Escherichia coli
Dr. Md. Sirazum Munir1, Dr. Nooriya Haque*2, Dr. Fahima Sultana3, Dr. Tarafder Mohammad Atiquzzaman4, Dr. Ahsanul Haque5
1Dr. Md. Sirazum Munir, Anesthesiologist, Intensive Care Unit Laboratory, Rajshahi Medical College Hospital, Rajshahi, Bangladesh
2Dr. Nooriya Haque, Assistant Professor (C.C), Department of Microbiology, Green Life Medical College, Dhaka, Bangladesh
3Dr. Fahima Sultana, Assistant Registrar, Department of Cardiology, Rajshahi Medical College Hospital
4Dr. Tarafder Mohammad Atiquzzaman, Medical Officer, Department of Paediatric Surgery, Bangladesh Medical University, Dhaka, Bangladesh
5Dr. Ahsanul Haque, Medical Officer, Department of Microbiology, Rajshahi Medical College, Rajshahi
*Corresponding author: Dr. Nooriya Haque, Assistant Professor (C.C), Department of Microbiology, Green Life Medical College, Dhaka, Bangladesh
Received: 21 July 2025; Accepted: 30 July 2025; Published: 07 August 2025
Article Information
Citation: Dr. Md. Sirazum Munir, Dr. Nooriya Haque, Dr. Fahima Sultana, Dr. Tarafder Mohammad Atiquzzaman, Dr. Ahsanul Haque. Generation of Protective Antibodies in Mice via Inactivated Whole-Cell Vaccine Targeting Multidrug-Resistant Escherichia coli. Fortune Journal of Health Sciences. 8 (2025): 768-773
Share at FacebookAbstract
Background: The growing prevalence of multidrug-resistant (MDR) Escherichia coli poses a significant challenge for clinicians, often leading to treatment failures. Developing an effective vaccine could help address issues related to antibiotic resistance and therapeutic limitations.
Objectives: This study aimed to evaluate the immune response elicited by a formaldehyde-inactivated MDR E. coli vaccine in a murine model.
Methods: A total of fifteen Swiss albino mice were used. The experimental group received three intradermal doses of formaldehyde-inactivated MDR E. coli at 14-day intervals. Following immunization, mice were challenged intraperitoneally with live E. coli and monitored for 14 days. Blood was collected from the tail to measure serum antibody binding using ELISA.
Results: All immunized mice (100%) survived the 14-day post-challenge period. ELISA results revealed a statistically significant increase (p < 0.001) in serum IgG levels both before and after the challenge in the immunized group.
Conclusion: This study demonstrates that formaldehyde-inactivated MDR E. coli can stimulate the production of protective antibodies in mice, suggesting its potential as a vaccine candidate.
Keywords
MDR E. coli, ELISA, Immune response, Whole cell vaccine
Article Details
Introduction
Antimicrobial resistance (AMR) has emerged as a critical global health issue, threatening the effectiveness of treatments for common infectious diseases and undermining decades of medical progress. The World Health Organization classifies AMR among the top ten global public health threats, emphasizing the urgent need for innovative strategies to combat resistant pathogens (1). The global pattern of antibiotic resistance varies due to genetic diversity among bacterial strains and differences in antibiotic accessibility and usage frequency across regions (2). Nevertheless, a common global trend is the significant rise in antimicrobial resistance (AMR) and multidrug resistance (MDR) among uropathogens responsible for community-acquired urinary tract infections (UTIs), which has considerably narrowed the spectrum of effective oral treatment options (3). Among these pathogens, Escherichia coli (E. coli) is of particular concern due to its role in a wide range of infections and its increasing resistance to multiple antibiotics, including last-resort treatments like carbapenems. Recent surveillance studies have highlighted a worrying trend: a significant proportion of carbapenem-resistant E. coli strains do not produce traditional carbapenemase enzymes, suggesting alternative resistance mechanisms that complicate both detection and treatment (4). The growing diversity and adaptability of E. coli strains call for urgent development of non-antibiotic interventions, particularly vaccines that can prevent infection or reduce bacterial burden.
Efforts to develop an effective vaccine against E. coli have been met with numerous challenges, primarily due to the pathogen’s genetic variability and complex interactions with the host immune system (5). Nonetheless, advances in immunological research have demonstrated that whole-cell inactivated vaccines can stimulate robust humoral and cellular responses, offering protection against a variety of Gram-negative bacteria (6,7). Formalin and heat-inactivated bacterial preparations, in particular, have shown promise in preclinical studies involving pathogens such as Citrobacter freundii and Klebsiella pneumoniae, leading to significant antibody production and protection in murine models (8). In this context, the present study investigates the immunogenicity and protective efficacy of a formaldehyde-inactivated whole-cell vaccine derived from a multidrug-resistant (MDR) E. coli strain in mice. By evaluating the generation of specific IgG antibodies and assessing protection in a controlled murine model, this work aims to contribute to the development of practical vaccine-based strategies to curb MDR E. coli infections.
Study Material and Methods
This in vivo study was conducted in the department of Microbiology of Dhaka Medical College and was approved by the ethical review committee.
Animals
Fifteen 4-6 weeks old Swiss albino female mice were obtained from the Animal Resources Facility of ICDDRB and reared under specific pathogen-free (SPF) conditions and maintained in the animal house of the Department of Microbiology, Dhaka Medical College. The mice were randomly distributed into 3 groups. The experimental mice belong to the experimental group (group-1) (5 mice), the control group (group-2) (5 mice), and the negative control group (group-3) (5 mice). Group 1 was immunized with formaldehyde-inactivated whole cells of Esch. coli, group-2 received PBS and group-3 was left uninoculated, uninfected.
Immunization of Mice
Bacterial Culture and CFU Determination
Previously isolated MDR E. coli strains were collected from various clinical samples and selected as candidates for inactivated whole-cell vaccine preparation. All bacterial strains were subcultured onto Mueller-Hinton agar plates and incubated at 37°C for 24 hours before each use. This ensured that the bacteria were in the same growth stage across all experimental steps.
Preparation of Formalin-Inactivated Whole-Cell E. coli
A loopful of E. coli was inoculated into Trypticase Soy Broth (TSB) in a microcentrifuge tube and incubated overnight at 37°C. After incubation, the bacterial cultures were centrifuged at 2,000 g for 20 minutes at 4°C, and the supernatant was discarded. The pelleted bacteria were then washed twice using ice-cold phosphate-buffered saline (PBS) (2,000 g, 20 minutes, 4°C). To prepare the formalin-inactivated E. coli whole-cell vaccine, 37% formalin was added to the suspension to achieve a final concentration of 3% (v/v). Specifically, 8.11 mL of 37% formalin was mixed with 91.89 mL of sterile distilled water. The suspension in the microcentrifuge tube was then incubated for 2 hours at 37°C. After incubation, the suspension was washed twice more with sterile ice-cold PBS (2,000 g, 20 minutes, 4°C) and resuspended in ice-cold sterile PBS to reach a concentration of 1.5 x 10^8 CFU/mL. Subsequently, 134 µL of this inoculum was mixed with 866 µL of sterile PBS in another microcentrifuge tube to achieve a concentration of 2X107 CFU/mL. Complete inactivation of the bacteria was confirmed by streaking onto Mueller-Hinton agar plates and observing no growth after overnight incubation at 37°C. The inactivated bacteria were then stored at -20°C until inoculation (6, 9).
Immunization Schedule
Three intradermal (I/D) inoculations were performed using 250 µL of bacterial solution (2X107 CFU/mL) on days 0, 14, and 28 in the alternate thighs of the experimental group (Group 1) of mice, which received the formalin-inactivated whole-cell E. coli. The control group (Group 2) of mice received 250 µL of sterile PBS on the same schedule. Intradermal inoculation was carried out using an insulin syringe (BD Ultra-Fine™ 31G). The mixtures were inoculated after providing proper anesthesia via an intraperitoneal injection of ketamine, adjusted according to the body weight of the mice (100 mg/kg) (10).
Serum Collection for ELISA
Blood samples were obtained from the tail veins of mice at specified intervals: 10 days following the initial immunization and subsequently 7 days after each booster dose. Before collection, mice were anesthetized using ketamine to ensure minimal discomfort. The tail was then cleansed with 70% ethanol. A sterile scalpel (22 FR) was employed to make a small incision approximately 2 mm from the tail tip. An initial 10 µL of blood was collected into a microcentrifuge tube containing 40 µL of phosphate-buffered saline (PBS), achieving a 1:5 dilution ratio. Hemostasis was achieved by applying sterile cotton to the incision site for approximately 5 minutes. The diluted blood samples were allowed to stand upright at room temperature for 2 hours to facilitate clotting, followed by centrifugation at 3,000 × g for 10 minutes. The resultant serum supernatant was carefully transferred to sterile microcentrifuge tubes and stored at −20°C for subsequent analysis (8).
Intraperitoneal Challenge
Two weeks post-final immunization, mice from both the experimental (Group 1) and control (Group 2) cohorts were subjected to an intraperitoneal challenge. Each mouse received an injection of 300 µL PBS containing 2 × 10^8 CFU/mL of live multidrug-resistant E. coli. Post-challenge, mice were monitored daily over a 14-day period for clinical signs indicative of infection, including weight loss, decreased activity, reduced food intake, and mortality.
2.6 Antibody Detection via ELISA
An indirect enzyme-linked immunosorbent assay (ELISA) was conducted to quantify IgG antibodies specific to E. coli antigens in mouse serum samples. Antigens were prepared through sonication of E. coli cultures. ELISA plates were coated with the prepared antigens, and serum samples were added in appropriate dilutions. Following incubation and washing steps, a secondary antibody conjugated to an enzyme was introduced. The optical density (OD) was measured at 450 nm using an ELISA plate reader (BioTek Inc., USA). The cut-off OD value was determined using the formula: Cut-off OD value = Mean OD + 2 × Standard Deviation. All data were systematically recorded for analysis.
Data Analysis
The data were documented systematically. All statistical analysis was performed using SPSS version 25. Differences between groups were compared with an unpaired t-test. A p-value of < .05 was considered the minimum level of significance.
Results
Bacterial Inactivation Using Formaldehyde
To assess the efficacy of formaldehyde in bacterial inactivation, treated bacterial suspensions were incubated overnight on Mueller-Hinton agar plates. Post-incubation, no bacterial colonies were observed, indicating successful inactivation. In contrast, control samples containing untreated bacteria exhibited normal growth patterns, confirming the viability of the bacteria before treatment.
Murine Survival Post-Lethal Bacterial Challenge
Following a lethal challenge, all mice in the experimental group (Group 1) survived for up to 14 days post-challenge, resulting in a 100% survival rate. Conversely, all mice in the control group (Group 2) succumbed within 24 hours, underscoring the protective effect conferred by the experimental intervention.
Humoral Immune Response Against E. coli
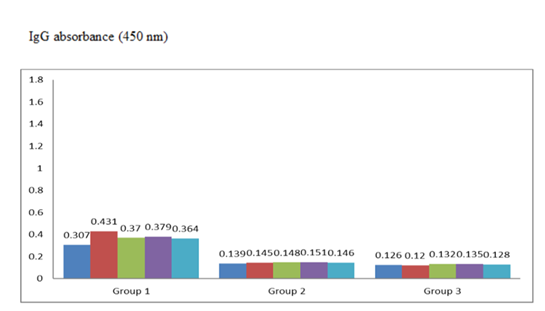
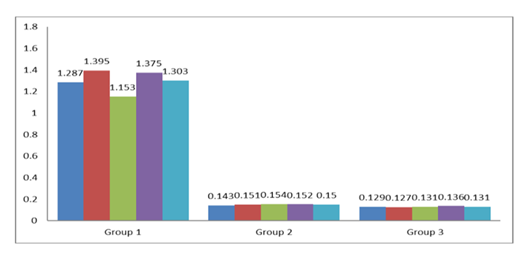
Peripheral blood samples were collected from the tail vein of mice at multiple time points: 10 days after the initial immunization and 7 days after each subsequent immunization, and the lethal challenge. Enzyme-linked immunosorbent assay (ELISA) was used to quantify anti-E. coli IgG polyclonal antibodies in the sera. OD of anti- E. coli antibodies in 10 serum samples (5 were from Group 1 and 5 were from Group 2 mice) collected 10 days after the first booster immunization [Figure 1] and in 10 serum samples (5 were from Group 1 and 5 were from Group 2 mice) collected 7 days after the second booster [Figure 2] was determined. All the serum samples from immunized mice had an OD of anti-E. coli above the cutoff value of 0.140 after the first booster and 0.137 after the second booster, respectively. There was a statistically significant difference between the OD values of experimental and control mouse sera (P = 0.0003 after the first booster and P = 0.00001 after the second booster).
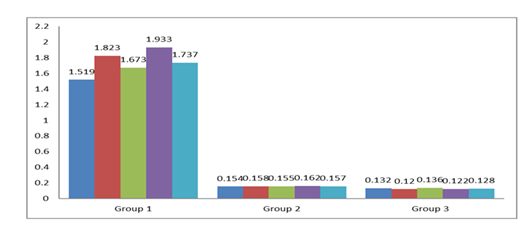
OD of anti-E. coli antibodies in serum samples collected after the lethal challenge are shown in Figure 3. Among 10 serum samples, 5 were from Group 1 and 5 were from Group 2 mice. All the serum samples had an OD of anti-E. coli IgG polyclonal antibody above the cutoff value of 0.142. The difference between the OD values of experimental and control mouse sera was statistically significant (P = 0.00002).
Table 1 presents the optical density values of IgG absorbance (450 nm) across different inoculation schedules within the experimental group, as analyzed by a one-way ANOVA. The calculated F value (130.56842) exceeded the critical F value (3.23887) from the F distribution table, and the P value was less than 0.0001, indicating statistically significant differences. Therefore, significant differences were observed in the serum IgG antibody titers of the experimental group of mice at various stages of the vaccine inoculation schedule.
Table 1: OD Value Differences in Immune Response Following Each Inoculation of Formalin-Inactivated Vaccine Analyzed by ANOVA
|
Sum of squares |
Df |
Mean square |
F value |
F crit |
P value |
|
|
Between group |
5.08695 |
3 |
1.695649 |
130.56842 |
3.23887 |
<0.0001 |
|
Within group |
0.20779 |
16 |
0.012987 |
|||
|
Total |
5.29474 |
19 |
Discussion
The intricate mechanisms underlying the pathogenesis of Escherichia coli and the rising prevalence of multidrug-resistant (MDR) strains contribute significantly to the increased vulnerability of certain populations to infections by this pathogen. Consequently, vaccine development presents a promising alternative to antibiotic therapy (11). In the current investigation, complete survival (100%) was observed in immunized mice subjected to a lethal bacterial challenge. A previous study demonstrated a 100% to 50% survival rate in mice immunized intramuscularly with PcrVNH—an engineered derivative of the PcrV protein, which is a component of the type III secretion system in Pseudomonas aeruginosa—between 36 hours and 7 days post-infection (12). However, there is currently no available data from Bangladesh regarding survival outcomes following lethal exposure to live MDR E. coli in murine models.
To enhance immune recognition, a whole-cell vaccine approach was employed, facilitating broad antigen presentation and thereby eliciting a more robust and comprehensive immune response. Formaldehyde was selected for bacterial inactivation due to its ability to preserve surface antigen integrity, which is crucial for effective immunogenicity (11,12). In this study, immunization with formaldehyde-inactivated whole-cell preparations successfully induced production of IgG polyclonal antibodies against E. coli, as detected by ELISA, both after immunization and subsequent lethal challenge. Comparable findings from a study in China indicated that formaldehyde-inactivated bacteria induced both IgG1 and IgG2a subclasses, with peak antibody titers observed on day 56 post-initial immunization (10). Similarly, another investigation reported that heat-inactivated whole-cell E. coli elicited significant systemic (serum IgG and IgM) and mucosal (lung IgA) antibody responses, demonstrating both mucosal and humoral immune activation (13). In the current study, the highest optical density (OD) values for IgG antibodies were recorded following the administration of the second booster, likely due to enhanced memory B cell activation and IgG production. A slight reduction in IgG levels was noted after the lethal challenge, potentially reflecting the clearance of the pathogen from circulation (14).
In this study, formaldehyde-inactivated E. coli strains isolated from urinary tract infection cases were used to immunize five Swiss albino mice (Group 1), followed by exposure to a lethal challenge in both the experimental (Group 1) and control (Group 2) groups. Notably, all immunized mice survived the 14-day post-challenge period. Similar results have been documented in Iran, where intradermal administration of formalin-inactivated E. coli led to 100% survival in mice (6). The use of formaldehyde-inactivated whole-cell bacteria in this study was aimed at maximizing antigenic diversity, thereby stimulating a more extensive and effective immune response.
Conclusion
The urgent need for an effective vaccine against this pathogen stems from its potential to lower infection rates, alleviate the burden on high-risk populations, and reduce the selective pressure driving antibiotic resistance. While further studies involving larger animal models are necessary to fully assess the immune response, the current findings offer encouraging evidence that a formaldehyde-inactivated whole-cell vaccine targeting MDR E. coli could provide protective immunity in humans.
Ethical Clearance
Ethical approval for this study was granted by the Institutional Review Board.
Financial Support and Sponsorship
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Health Organization. 10 global health issues to track in 2021 (2020).
- Pakzad I, Reza Mortazavi-Tabatabaei S, Ghaderkhani J, et al. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int J Prev Med 10 (2019): 169.
- Frazee BW, Trivedi T, Montgomery M, et al. Emergency Department Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae: Many Patients Have No Identifiable Risk Factor and Discordant Empiric Therapy Is Common. Annals of Emergency Medicine 72 (2018): 449–56.
- Cienfuegos-Gallet A, Ocampo AM, Chavda K, et al. Molecular epidemiology of carbapenem-resistant Enterobacter cloacae complex infections uncovers high frequency of non-carbapenemase-producers in five tertiary care hospitals from Colombia. Epidemiology (2018).
- Sainz-Mejías M, Jurado-Martín I, McClean S. Understanding Pseudomonas aeruginosa–Host Interactions: The Ongoing Quest for an Efficacious Vaccine. Cells 9 (2020): 2617.
- Fan Y, Mu Y, Lu L, et al. Hydrogen peroxide-inactivated bacteria induces potent humoral and cellular immune responses and releases nucleic acids. International Immunopharmacology 69 (2019): 389–97.
- Rahman M, Shukla SK, Prasad KN, et al. Prevalence and molecular characterisation of New Delhi metallo-β-lactamases NDM-1, NDM-5, NDM-6 and NDM-7 in multidrug-resistant Enterobacteriaceae from India. International Journal of Antimicrobial Agents 44 (2014): 30–7.
- Kawser Z, Shamsuzzaman SM. Intradermal Immunization with Heat-Killed Klebsiella pneumoniae Leading to the Production of Protective Immunoglobulin G in BALB/c Mice. Int J Appl Basic Med Res 11 ( 2021): 160–5.
- Takahashi K, Hanamura Y, Tokunoh N, et al. Protective effects of oral immunization with formalin-inactivated whole-cell Citrobacter rodentium on Citrobacter rodentium infection in mice. Journal of Microbiological Methods 159 (2019): 62–8.
- Fan Y, Mu Y, Lu L, et al. Hydrogen peroxide-inactivated bacteria induces potent humoral and cellular immune responses and releases nucleic acids. International Immunopharmacology 69 (2019): 389–97.
- Crothers JW, Norton EB. Recent advances in enterotoxin vaccine adjuvants. Current Opinion in Immunology 85 (2023): 102398.
- Wan C, Zhang J, Zhao L, et al. Rational Design of a Chimeric Derivative of PcrV as a Subunit Vaccine Against Pseudomonas aeruginosa. Front Immunol 24 (2019): 10.
- Arshadi N, Mousavi SL, Amani J, et al. Immunogenic Potency of Formalin and Heat Inactivated E. coli O157:H7 in Mouse Model Administered by Different Routes. Avicenna J Med Biotechnol 12 (2020): 194–200.
- Kawser Z, Shamsuzzaman SM. “Intradermal immunization with heat-killed Klebsiella pneumoniae Leading to the production of protective immunoglobulin G in BALB/c mice”, International Journal of Applied & Basic Medical Research 11 (2021): 160-165.