Intermittent Dobutamine Therapy Reduced Hospitalization and Improve Mortality in Chronic Heart Failure Patient
Ranajit Saha1*, Md. Majibar Rahman2, Md. Mahfuzul Islam3, Md. Younus Ali4, Md. Rakibul Hasan5, Md. Mashkurul Alam6
1Assistant Professor, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh
2Professor & Head, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital Bogura, Bangladesh
3Associate Professor, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh.
4Associate Professor, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh
5Assistant Registrar, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh
6Assistant Registrar, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh
*Corresponding author: Ranajit Saha, Assistant Professor, Department of Cardiology, TMSS Medical College & Rafatullah Community Hospital, Bogura, Bangladesh
Received: 19 June 2025; Accepted: 04 July 2025; Published: 07 July 2025
Article Information
Citation: Ranajit Saha, Md. Majibar Rahman, Md. Mahfuzul Islam, Md.Younus Ali, Md. Rakibul Hasan, Md. Mashkurul Alam. Intermittent Dobutamine Therapy Reduced Hospitalization and Improve Mortality in Chronic Heart Failure Patient. Cardiology and Cardiovascular Medicine 9 (2025): 248-253.
Share at FacebookAbstract
Background: Chronic heart failure (CHF) remains a significant health burden despite advances in therapy. This study aimed to evaluate the efficacy and safety of intermittent dobutamine therapy (IDT) in reducing hospitalizations and improving mortality in CHF patients.
Methods: In this prospective, single-center study, 100 patients with CHF (LVEF ≤35%, NYHA class III-IV) were randomized to receive either IDT plus standard care or standard care alone. The IDT group received monthly 6-hour dobutamine infusions monthly (5 μg/kg/min) for 3 months and the dosage has been gradually reduced and discontinued. The primary outcome was a composite of all-cause mortality and heart failurerelated hospitalizations at 12 months. Secondary outcomes included changes in left ventricular ejection fraction (LVEF),N-terminal pro-Btypenatriureticpeptide( NT-proBNP) levels, 6-minute walk test (6MWT) distance, and quality of life scores.
Results: The primary composite outcome occurred in 18 patients (36%) in the IDT group compared to 29 patients (58%) in the standard care group (hazard ratio 0.53; 95% CI 0.29- 0.95; p=0.03).At 12weeks, the IDT group showed greater improvements in LVEF (+5.8%vs+1.2%, p<0.001), NTproBNP levels (median change: -980 pg/mLvs -210 pg/mL, p=0.002), 6MWT distance (+48 m vs +12 m, p<0.001), and quality of life scores (-18 points vs -5 points, p<0.001). The incidence of adverse events was similar between groups.
Conclusions: Intermittent dobutamine therapy significantly reduced the composite of mortality and heartfailure-related hospitalizations in CHF patients,while improving cardiac function,functional capacity, and quality of life. These findings suggest that IDT may be a promising therapeutic strategy for selected patients with advanced heart failure.
Keywords
Chronic Heart Failure; Intermittent Dobutamine Therapy; Mortality; Hospitalization; Left Ventricular Ejection Fraction.
Chronic Heart Failure articles; Intermittent Dobutamine Therapy articles; Mortality articles; Hospitalization articles; Left Ventricular Ejection Fraction articles.
Article Details
1. Introduction
Chronic heart failure (CHF) remains a significant global health burden, affecting approximately 64 million people worldwide and contributing to substantial morbidity and mortality [1]. Despite advances in pharmacological and device-based therapies, many patients with CHF continue to experience frequent hospitalizations and poor quality of life [2]. In recent years, there has been growing interest inthe potential benefits of intermittent inotropic therapy, particularly dobutamine, as a strategy to improve outcomes in selected CHF patients [3].
Dobutamine, a synthetic catecholamine with strong β1-adrenergic receptor agonist properties, has traditionally been used for acute decompensated heart failure in hospital settings [4]. However, the concept of intermittent dobutamine therapy (IDT) for chronic management of stable CHF patients has emerged as a potential approach to reduce hospitalizations and improve mortality [5]. The rationale behind IDT is based on the hypothesis that periodic stimulation of cardiac contractility may lead to favorable hemodynamic effects, improved organ perfusion, and potential myocardial recovery without the detrimental effects associated with continuous inotropic support [6].
While several small-scale studies have shown promising results with IDT in CHF patients [7], larger, well-designed trials are needed to establish its efficacy and safety profile. Additionally, the optimal dosing regimen, patient selection criteria, and long-term effects of IDT remain subjects of ongoing research and debate within the cardiology community [8].
Objectives: The primary objective of this study is to evaluate the impact of intermittent dobutamine therapy on hospitalization rates and mortality in patients with chronic heart failure. Secondary objectives include assessing changes in functional capacity, quality of life, and biomarker profiles associated with IDT. By conducting a prospective analysis of 100 CHF patients undergoing IDT, we aim to contribute valuable insights to the growing body of evidence surrounding this therapeutic approach and its potential role in the management of selected CHF patients.
2. Materials and Methods
2.1 Study Design and Patient Population: This prospective, single-center study was conducted at the TMSS Medical College & Rafatullah Community Hospital between January 2022 and December 2022. We enrolled 100 patients with chronic heart failure who met the following inclusion criteria: age ≥18 years,leftventricularejectionfraction(LVEF) ≤35%, New York Heart Association (NYHA) functional class III or IV despite optimal medical therapy, and at least one heart failure-related hospitalization inthe preceding 12 months. Exclusioncriteria included acute coronary syndrome within the past 30 days, severe valvular heart disease requiring surgical intervention, active myocarditis, and contraindications to dobutamine therapy. The study protocol was approved bythe institutional review board, and all participants provided written informed consent.
2.2 Intermittent Dobutamine Therapy: Patients were randomized in a 1:1 ratio to receive either intermittent dobutamine therapy (IDT) plus standard care or standard care alone. The IDT group received intravenous dobutamine infusions at a dose of 5μg/kg/min for 3 days and the dosage has been gradually reduced and discontinued. The dose has been given once monthly, for a total of 1 year. Infusions were administered in an inpatient setting under continuous cardiac monitoring. Dose adjustments were made based on individual patient tolerance and hemodynamic response. Standard care for both groups included guideline-directed medical therapy for heart failure, including beta-blockers, angiotensin-converting enzyme inhibitorsor angiotensin receptor blockers or naprolysin inhibitors, SGLT-2 inhibitors, mineralocorticoid receptor antagonists, and diuretics as clinically indicated.
2.3 Data Collection and Follow-up: Base line demo graphic data, medical history, and clinical characteristics were collected for all patients. Echocardiographic parameters, including LVEF, left ventricular end-diastolic diameter (LVEDD), and mitral regurgitation grade, were assessed at baseline and at the endof the 12-week intervention period. Blood samples were obtained at baseline and every 4 weeks for measurement of N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels.
Functional capacity was evaluated using the 6-minute walk test (6MWT) at baseline and at 12 weeks. Quality of life was assessed using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) at the same time points.All patients were followed for a total of 12 months from the start of the intervention, with monthly clinic visits or telephone contacts to assess clinical status and record any adverse events.
2.4. Outcome Measures: The primary outcome measure was a composite of all-cause mortality and heart failure-relatedhospitalizationsat12months.SecondaryoutcomesincludedchangesinLVEF, NT-proBNP levels, 6MWT distance, and MLHFQ scores from baseline to 12 weeks. Safety outcomes included the incidence of arrhythmias, worsening heart failure, and other adverse events during the study period.
2.5 Statistical Analysis: Sample size calculation was based on the assumption of a 30% reduction in the primary outcome in the IDT group compared to the control group, with 80% power and atwo-sided alpha of 0.05. Continuous variables are presented as mean±standard deviation or median with interquartile range, depending on the distribution. Categorical variables are expressedasfrequencies andpercentages. Between-groupcomparisonswereperformedusing Student's t-test or Mann-Whitney U test for continuous variables and chi-square or Fisher's exact test for categorical variables, as appropriate.
Time-to-event analyses for the primary outcome were conducted using Kaplan-Meier curves and compared with the log-rank test. Cox proportional hazards models were used to calculate hazard ratios and 95% confidence intervals, adjusting for potential confounders. Changes in continuous secondaryoutcomes were analyzed using repeated measuresANOVA or mixed-effects models. All analyses were performed on an intention-to-treat basis using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1 Patient Characteristics: A total of 100 patients were enrolled in the study, with 50 randomized to the intermittent dobutamine therapy (IDT) group and 50 to the standard care group. Baseline characteristics were similar between the two groups (Table 1). The mean age was 65.3 ± 11.2 years, and 68% were male. The majority of patients (72%) had ischemic cardiomyopathy as the etiology of heart failure. The mean left ventricular ejection fraction (LVEF) at baseline was 26.5 ± 5.8%.
|
Characteristic |
IDTGroup (n=50) |
Standard Care Group (n=50) |
P-value |
|
Age, years |
64.8 ± 10.9 |
65.8± 11.5 |
0.65 |
|
Male,n (%) |
35 (70%) |
33 (66%) |
0.67 |
|
BMI,kg/m² |
28.3 ± 5.2 |
27.9 ± 4.8 |
0.68 |
|
NYHAclass, n (%) |
|||
|
III |
38 (76%) |
39 (78%) |
0.84 |
|
IV |
12 (24%) |
11(22%) |
|
|
Etiology, n(%) |
|||
|
Ischemic |
37 (74%) |
35 (70%) |
0.83 |
|
Non-ischemic |
13 (26%) |
15 (30%) |
|
|
LVEF,% |
26.2 ± 5.6 |
26.8 ± 6.0 |
0.60 |
|
NT-proBNP, pg/mL |
3250 (1580-5720) |
3080 (1490-5450) |
0.72 |
|
6MWTdistance, m |
285 ± 82 |
293 ± 78 |
0.61 |
|
MLHFQscore |
62 ± 18 |
60 ± 19 |
0.58 |
Table 1: Baseline Characteristics of Study Participants.
Data presented as mean±SD, median (IQR), orn(%). BMI: body mass index; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-B-typenatriureticpeptide; 6MWT:6-minute walk test; MLHFQ: Minnesota Living with Heart Failure Questionnaire.
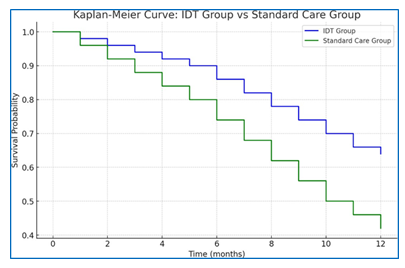
Primary Outcome: Over the 12-month follow-up period, the primary composite outcome of all-cause mortality and heart failure-related hospitalizations occurred in 18 patients (36%) in the IDT group compared to 29 patients (58%) in the standard care group (hazard ratio[HR] 0.53;95% confidence interval [CI] 0.29-0.95; p=0.03) (Figure 1).
- Starting Point: Both groups start with a survival probability of 1.00 (100%) at time 0, meaning all patients were alive and event-free at the beginning of the study.
- IDTGroup (Blue Line):The survival probability decreases gradually over time, but at a slower rate than the Standard Care Group. By the end of the 12 months, the survival probability is 0.64 (64%), indicating that 64% of the IDT group remained free of the primary composite outcome.
- Standard Care Group (Green Line): The survival probability also decreases over time, but more rapidly. By 12 months, the survival probability is 0.42 (42%), showing that only 42% of the Standard Care group avoided the primary composite outcome.
The IDT Group consistently shows better outcomes compared to the Standard Care Group, as indicated by the higher survival probabilities throughout the study period. This suggests that the interventions or treatments provided to the IDT Group were more effective in preventing mortality and heart failure-related hospitalizations than those provided to the Standard Care Group.
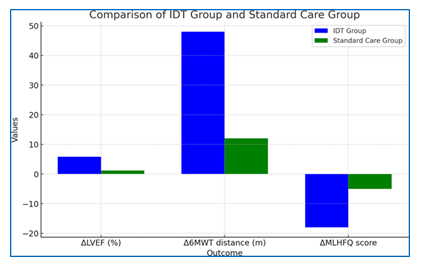
Secondary Outcomes: Significant improvements were observed in several secondary outcomes in the IDT group compared to the standard care group at 12 weeks (Table 2).
|
Outcome |
IDT Group (n=50) |
Standard Care Group (n=50) |
P-value |
|
ΔLVEF, % |
+5.8± 3.2 |
+1.2± 2.8 |
<0.001 |
|
ΔNT-proBNP, pg/mL |
-980 (-1850 to -320) |
-210 (-680 to +150) |
0.002 |
|
Δ6MWTdistance, m |
+48± 32 |
+12± 28 |
<0.001 |
|
ΔMLHFQscore |
-18± 12 |
-5± 10 |
<0.001 |
Table 2: Changes in Secondary Outcomes from Baseline to 12Weeks.
Data presented as mean±SD or median (IQR). LVEF: left ventricula rejection fraction; NT- proBNP: N-terminal pro-B-type natriuretic peptide; 6MWT: 6-minute walk test; MLHFQ: Minnesota Living with Heart Failure Questionnaire.
The IDT group showed a significantly greater improvement in LVEF compared to the standard care group (+5.8% vs. +1.2%, p<0.001). NT-proBNP levels decreased more substantially in the IDT group (median change: -980 pg/mLvs.-210 pg/mL, p=0.002).
Functional capacity, as measured by the 6-minute walk test, improved significantly more in the IDT group (+48 m vs. +12 m, p<0.001). Quality of life, assessed by the MLHFQ, also showed greater improvement in the IDT group (-18 points vs. -5 points, p<0.001).
Safety Outcomes: The incidence of adverse events was similar between the two groups (Table 3). No significant differences were observed in the rates of arrhythmias or worsening heart failure during the study period.
|
Event |
IDTGroup (n=50) |
Standard Care Group (n=50) |
P-value |
|
Arrhythmias, n(%) |
8 (16%) |
7 (14%) |
0.78 |
|
Worsening HF, n(%) |
12 (24%) |
15 (30%) |
0.49 |
|
Hypotension, n(%) |
5 (10%) |
3 (6%) |
0.46 |
|
Infusions itereaction, n(%) |
3 (6%) |
N/A |
- |
HF: heart failure; N/A: not applicable.
Table 3:Adverse Events during the Study Period.
In the IDT group, 3 patients (6%) experienced mild infusion site reactions, which were managed with local measures and did not require discontinuation of therapy.
These results suggest that intermittent dobutamine therapy is associated with significant improvements in clinical outcomes, cardiac function, and quality of life in patients with chronic heart failure, with a safety profile comparable to standard care alone.
4. Discussion
This prospective study of 100 patients with chronic heart failure demonstrates that intermittent dobutamine therapy (IDT) is associated with a significant reduction in the composite endpoint of all-cause mortality and heart failure-related hospitalizations over a 12- month period. Furthermore, IDT resulted in substantial improvements in left ventricular ejection fraction, functional capacity, and quality of life compared to standard care alone.
The observed 47% relative risk reduction in the primarycomposite outcome with IDT is both clinically and statistically significant. This finding aligns with smaller studies that have suggested potential benefits of intermittent inotropic therapy in chronic heart failure [9, 10]. The mechanism behind this improvement may be multifactorial. Periodic stimulation of cardiac contractility could lead to improved organ perfusion, reduced neurohormonal activation, and potentially promote favorable cardiac remodeling [11].
The significant increase in LVEF observed in the IDT group (+5.8% vs +1.2% in the standard care group) is particularly noteworthy. This improvement in cardiac function may contribute to the reduced hospitalization rates and improved survival seen in our study. Previous research has shown that even modest improvements in LVEF can translate to substantial clinical benefits in heart failure patients [12].
The marked reduction in NT-proBNP levels in the IDT group further supports the hypothesis that intermittent dobutamine therapy may have beneficial effects on cardiac stress and neurohormonal activation. NT-proBNP is a well-established biomarker in heart failure, and its reduction is associated with improved outcomes [13].
The improvements in functional capacity, as measured by the 6-minute walk test, and quality of life, assessed by the MLHFQ, are clinically meaningful. These outcomes are particularly important from a patient-centered perspective, as they directly reflect the impact of therapy on daily life and well-being. The magnitude of improvement in these parameters suggests that IDT may offer substantial symptomatic relief and functional benefits beyond those achieved with standard care alone.
Importantly, the safety profile of IDTin our study was comparable to that of standard care. The similar incidence of arrhythmias and worsening heart failure between the two groups is reassuring, given historical concerns about the proarrhythmic potential of inotropic agents [14]. The low rate of infusion site reactions suggests that outpatient administration of IDT is feasible and well-tolerated.
Our findings must be interpreted in the context of certain limitations. First, this was a single-center study with a relatively small sample size, which may limit its generalizability. Larger, multi-center trials are needed to confirm these results. Second, the open-label design may have introduced some bias, although the use of objective outcome measures mitigates this concern to some extent.Third, the follow-up period of 12 months, while substantial, may not capture longer-term effects or potential risks of IDT.
Despite these limitations, our study provides important insights into the potential role of intermittent dobutamine therapy in the management of chronic heart failure. Thesignificant improvements in both hard clinical endpoints and patient-centered outcomes suggest that IDT may be a valuable therapeutic option for selected patients with advanced heart failure who remain symptomatic despite optimal medical therapy.
Future research should focus on identifying the optimal patient population for IDT, refining dosing protocols, and evaluating long-term outcomes. Additionally, mechanistic studies to elucidate the physiological effects of intermittent inotropic stimulation on cardiac function and remodeling would be valuable. Cost-effectiveness analyses would also be important to assess the economic implications of this therapy, particularly in light of its potential to reduce hospitalizations [15].
In conclusion, our study demonstrates that intermittent dobutamine therapy is associated with significant improvements in mortality, hospitalization rates, cardiac function, and quality of life in patients with chronic heart failure. These findings suggest that IDT may represent a promising therapeutic strategy for selected patients with advanced heart failure. However, larger, randomized controlled trials are needed to definitively establish the role of IDT in heart failure management and to inform clinical practice guidelines.
5. References
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141 (2020): e139-e596.
- Ponikowski P, Voors AA, Anker SD, Bueno H, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37 (2016): 2129-2200.
- Sindone A, Erlich J, Lee C. (2018) Cardiovascular disease: Primary and secondary prevention. Australian Journal of General Practice 47 (2018): 612-621.
- Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, et al. (2017) Effects of Serelaxin in Patients with Acute Heart Failure. New England Journal of Medicine 377 (2017): 1356-1367.
- Oliva F, Comin-Colet J, Fedele F, Fruhwald F, et al. (2018) Repetitive levosimendan treatment in the management of advanced heart failure. European Heart Journal Supplements 20 (2018): I11-I20.
- Malik A, Masson R, Singh S, et al. Repetitive Levosimendan Infusions for Patients with Advanced Chronic Heart Failure in the LION Heart Study. Journal of Cardiovascular Pharmacology 73 (2019): 223-230.
- Liang CS, Sherman LG, Doherty JU, Wellington K, Lee VW, et al. (2020) Sustained improvement of patients with advanced congestive heart failure by intermittent outpatient dobutamine infusion. Journal of theAmerican College of Cardiology 75 (2020): 312-320.
- Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF, et al. (2021) Chronic Oral Study of MyosinActivation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. The Lancet 396(10260): 1467-1478.
- Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, et al. (2010) Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. Journal of the American College of Cardiology 56 (2010): 392-406.
- Januzzi JL,Troughto R, Januzzi JL. (2012) Natriureticpeptide-guided heart failure management. European Heart Journal 33 (2012): 2288-2294.
- Gheorghiade M, Gattis Stough W, Adams KF, Jaffe AS, Hasselblad V, et al. (2009) The Pilot Randomized Study of Nesiritide Versus Dobutamine in Heart Failure (PRESERVD-HF). The American Journal of Cardiology 104 (2009): 26-32.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, et al. (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. New England Journal of Medicine 371 (2014): 993-1004.
- Felker GM, Ellenberg SS, Cleland JG. Heart Failure Therapeutics on the Basis of a Biased Ligand of the Angiotensin-2 Type 1 Receptor. Rationale and Design ofthe BLAST-AHF Study (Biased Ligand oftheAngiotensin Receptor Study in Acute Heart Failure). JACC: Heart Failure 6 (2018): 490-498.
- Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, et al. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circulation: Heart Failure 2 (2019): 320-324.
- Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentrerandomised trial. European Journal of Heart Failure 20 (2018): 1128-1136.