Isolation and Characterization of Small Pluripotent Stem Cells (SPSCs) from Human Peripheral Blood: A Novel Cold-Enrichment Protocol
Torbjörn Ogéus DC, PgD, MSc, ScA* and Adam Roos DC
Stockholms Led- & Smärtklinik, Stockholm, Sweden
*Corresponding author: Torbjörn Ogéus, Stockholms Led- & Smärtklinik, Stockholm, Sweden.
Received: 28 July 2025; Accepted: 06 August 2025; Published: 21 August 2025
Article Information
Citation: Torbjörn Ogéus, Adam Roos. Isolation and Characterization of Small Pluripotent Stem Cells (SPSCs) from Human Peripheral Blood: A Novel Cold-Enrichment Protocol. Archives of Clinical and Biomedical Research. 9 (2025): 322-331.
Share at FacebookAbstract
Introduction: Small Pluripotent Stem Cells (SPSCs) represent a novel population of blood-derived stem cells exhibiting key pluripotency markers (OCT4, NANOG, SOX2, SSEA-4), measuring <5 μm in size. Isolated through a non-manipulated, cold-enrichment protocol from peripheral blood, SPSCs demonstrate nuclear integrity, high viability, and potential for both local and systemic therapeutic delivery. Unlike larger stem cells such as mesenchymal stem cells (MSCs), which are often sequestered in pulmonary capillaries, the small size of SPSCs may allow for wider biodistribution. Their autologous origin and compliance with FDA and EMA minimal manipulation guidelines make them an ideal candidate for future regenerative applications.
Methods: Peripheral blood samples (10 ml) were collected from healthy adult volunteers n=233 total, (200 for baseline, 23 for enrichment, 10 for staining) to be centrifuged according to the SBSC protocol published by Filidou et al. (2023). Cell concentration and viability were measured before and after cold storage using the LunaStem™ automated cell counter. Immunocytochemistry was performed using the Invitrogen™ Pluripotent Stem Cell Immunocytochemistry Kit (OCT4, SSEA-4, NANOG, SOX2). Images were captured using a Magus Lum D400 fluorescence microscope with MAGUS CLM30 digital camera.
Results: LunaStem™ analysis showed that the total nucleated cell concentration increased from ~20 million/ml to ~40 million/ml after 4.5h in cold storage, most prominently in the 1–3 μm size range. In multiple independent experiments for enrichment (n=23), a consistent ~2–3-fold increase in viable small cells (~1 μm) was observed. Pluripotent markers from separate blood samples (n=10) were shown and presented using immunofluorescence microscopy.
Conclusion: This study introduces a novel, minimally invasive method for isolating and enriching Small Pluripotent Stem Cells (SPSCs) from human peripheral blood. We observed a statistically significant 2-fold increase (p=0.0001) of pluripotent cells with yields ranging from 200–500 million SPSCs from 60 ml whole blood.
Keywords
Pluripotent stem cells; Peripheral blood; VSELs; SBSCs; Minimal manipulation; SSEA-4; OCT4; NANOG; SOX2; Cell size; Systemic biodistribution; Regenerative medicine; Cold enrichment; Autologous therapy, cold storage; Peripheral blood-derived stem cells
Pluripotent stem cells articles; Peripheral blood articles; VSELs articles; SBSCs articles; Minimal manipulation articles; SSEA-4 articles; OCT4 articles; NANOG articles; SOX2 articles; Cell size articles; Systemic biodistribution articles; Regenerative medicine articles; Cold enrichment articles; Autologous therapy articles, cold storage articles; Peripheral blood-derived stem cells articles.
Article Details
1. Introduction
Small Pluripotent Stem Cells (SPSCs) represent a novel population of blood-derived stem cells exhibiting key pluripotency markers (OCT4, NANOG, SOX2, SSEA-4), measuring <5 µm in size. Isolated through a non-manipulated, cold-enrichment protocol from peripheral blood, SPSCs demonstrate nuclear integrity, high viability, and potential for both local and systemic therapeutic delivery. Unlike larger stem cells such as mesenchymal stem cells (MSCs), which are often sequestered in pulmonary capillaries [1-3], the small size of SPSCs may allow for wider biodistribution. Their autologous origin and compliance with FDA and EMA minimal manipulation guidelines make them an ideal candidate for future regenerative applications [4-6]. This study reports a 2–3-fold increase in SPSCs after 4.5h of cold storage, with yields of 200–500 million cells from 60 ml blood, confirmed by immunofluorescence and viability assays.
Pluripotent stem cells (PSCs) are uniquely capable of differentiating into all three germ layers, ectoderm, mesoderm, and endoderm, offering regenerative potential for a wide range of tissues [7]. This property distinguishes them from multipotent stem cells, such as mesenchymal stem cells (MSCs), which are limited to specific lineages like bone, cartilage, and fat [8], and totipotent cells like the zygote, which can form both embryo and extra-embryonic tissues. While embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) exhibit robust plasticity, their clinical use is hindered by tumorigenic risks and complex ethical or regulatory considerations [9-11].
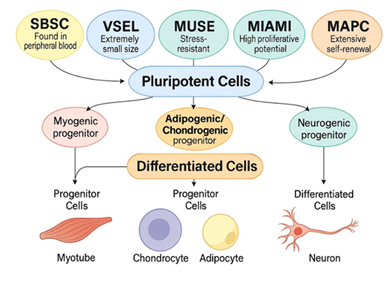
Stem cells can be classified into distinct potency categories based on their differentiation capacity: totipotent, pluripotent, and multipotent. Totipotent cells, such as the zygote and early embryonic blastomeres, have the unique ability to form all embryonic and extra-embryonic tissues, including the placenta. While totipotent cells hold theoretical regenerative promise, they are derived only from embryos and carry a substantial risk of tumorigenesis, including teratoma and teratocarcinoma formation [12,13]. Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can differentiate into cell types from all three germ layers, ectoderm, mesoderm, and endoderm, thus offering broad regenerative potential. However, ESCs and iPSCs also exhibit tumorigenic potential if not properly differentiated prior to transplantation [14,15]. In contrast, adult-derived pluripotent-like populations such as MUSE cells, VSELs, and SBSCs show trilineage differentiation capacity but have not demonstrated spontaneous tumor formation, making them safer alternatives for clinical use [16]. Multipotent stem cells, such as hematopoietic or mesenchymal stem cells, are restricted to specific lineages and cannot generate all cell types, which limits their regenerative versatility [17]. The distinction between these potency levels is critical in evaluating both the therapeutic potential and safety of various stem cell types.
Recent discoveries of endogenous, naturally occurring pluripotent-like cells in sub-populations within larger stem cell populations, including very small embryonic-like stem cells (VSELs) [18], multilineage-differentiating stress-enduring (Muse) cells [19], and small blood stem cells (SBSCs) [20], offer an exciting new avenue for regenerative therapies. These cell types are characterized by small size, expression of pluripotency markers (OCT4, NANOG, SOX2), and presence in adult tissues such as bone marrow, adipose tissue, or peripheral blood. Unlike iPSCs or ESCs, they arise naturally, are not genetically manipulated, and show minimal risk of teratoma formation, offering a safer and more accessible therapeutic option [21-23].
Additional candidates include MIAMI (Marrow-Isolated Adult Multilineage Inducible) cells [24] MAPCs (Multipotent Adult Progenitor Cells) which require extensive in vitro processing and must go through FACS-sorting for SSEA-3+ markers from mesenchymal stem cells [25], both showing pluripotent-like activity under defined conditions. These cells can differentiate into multiple tissue types while maintaining a favorable safety profile. Importantly, they may also be sourced from adult donors and processed under minimal manipulation guidelines, making them regulatory compliant when it comes to the autologous harvesting.
Among these, the cells described in this study, termed Small Pluripotent Stem Cells (SPSCs) represent a novel combination of VSEL- and SBSC-like populations isolated from peripheral blood through a non-enzymatic, non-manipulated protocol. These cells are <5 µm in diameter, viable, DAPI- and Hoechst-positive, and show consistent expression of key pluripotency markers (SSEA-4, OCT4, NANOG, SOX2). Their ability to increase markedly after 4.5h of cold storage suggests the presence of a mobilizable, quiescent stem cell population in human blood.
A major translational advantage of SPSCs is their small size. Most MSCs measure >15 µm and have been shown to become entrapped in pulmonary capillaries when administered intravenously, a phenomenon known as the “pulmonary first-pass effect” [1-3]. In contrast, SPSCs in the 1–5 µm range are unlikely to become trapped and may achieve systemic biodistribution. This could lead to greater therapeutic reach for diseases requiring systemic immune modulation or repair at distant sites [26].
Finally, because SPSCs are isolated without culture expansion, enzymatic treatment, or genetic alteration, they meet both EU (EMA) and FDA criteria for minimal manipulation and autologous harvesting, enabling potential clinical use under existing autologous use exemptions [4-6]. The combination of endogenous origin, pluripotency, systemic compatibility, and regulatory compliance places SPSCs in a promising position for further clinical development (Figure 1,2).
The aim of this study is to characterize the biological properties of SPSCs isolated from peripheral blood, including concentration changes after cold storage, viability, nuclear content, and marker expression. This data may support their future use in both local and systemic regenerative applications.
2. Materials and Methods
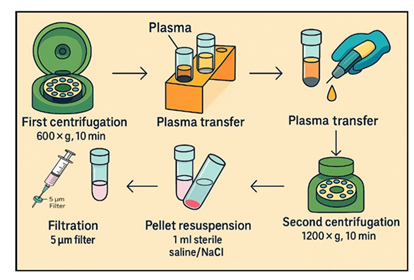
Peripheral blood samples (10 ml) were collected from healthy adult volunteers (n=200) into sodium citrate tubes and processed within 30 minutes of sampling. Samples were centrifuged at 600 × g for 10 minutes at room temperature using a horizontal swing-out bucket rotors centrifuge system (Bio-PRF, USA). The upper plasma layer was removed, placed in new vacuum tube and centrifuged for another 1200 × g for 10 minutes. After that, the pellets in the bottom of the tubes were retained, containing the putative small cell population. The cell pellet was resuspended in sterile 0.9% NaCl solution for further analysis according to the SBSC protocol published by Filidou et al. [20]. Cell concentration and viability were measured using the LunaStem™ automated cell counter. Acridine Orange/Propidium Iodide (AO/PI) was used to assess viability.
In the second stage of the study more peripheral blood samples (10 ml) were collected from healthy adult volunteers (n=23) into sodium citrate tubes and processed within one hour of sampling. Samples went through the same steps as the first 200 subjects, the pellets in the bottom of the tubes were retained, following the same protocol, but then later stored at 4°C in vacuum-sealed tubes without agitation for up to 4.5h (or 16–18h in select experiments).
Cell concentration and viability were measured before and after cold storage using the LunaStem™ automated cell counter. AO/PI was used to assess viability. Particular attention was given to cells within the 1–3 µm range.
To confirm the presence of nuclei in the smallest cells, samples were stained with DAPI and Hoechst 33342. These nuclear stains allowed confirmation of nuclear content in small cells that otherwise appeared nucleus-negative by LunaStem™. Additional samples going through the same protocol (n=10) were selected for dual DAPI/Hoechst staining, verifying consistent presence of nuclear material in the 1–3 µm fraction following the same protocol as with the SBSC cells [20].
Immunocytochemistry was also performed to these 10 samples using the Invitrogen™ Pluripotent Stem Cell Immunocytochemistry Kit (OCT4, SSEA-4, NANOG, SOX2). Cell pellets remained in the 10ml vacuum tubes during the staining protocol. Cells were fixed with 4% paraformaldehyde, permeabilized, and blocked per protocol. Primary antibodies were incubated overnight at 4°C. Fluorescent-labeled secondary antibodies were applied for 1 hour at room temperature in darkness.
After final washing, 10 μL of suspension was transferred to LunaStem™-compatible slides without coverslip. Images were captured using a Magus Lum D400 fluorescence microscope with a MAGUS CLM30 digital camera.
2.1 Statistical Analysis:
Mean and standard deviation (SD) or frequencies (percentage) were used to characterize the samples. Data normality was confirmed via Shapiro-Wilk tests (p > 0.05); if non-normality was detected, Wilcoxon signed-rank tests were used as a non-parametric alternative. Data comparisons between cell count groups were performed based on baseline and the cell count change with the use of paired t-tests for related samples. To investigate whether there were significant similarities between the increase groups (4.5h and 16h compared to baseline), paired t-tests or TOST were used for comparing fold increases. Post-hoc power was calculated using statsmodels (Python) assuming Cohen's f=0.4. All statistical tests were performed with Prism 10 for Windows (Microsoft, USA). For all statistical tests, the 0.05 level of probability was set as the criterion for statistical significance.
3. Results
3.1 Cell Enrichment During Cold Storage
LunaStem™ analysis of the first samples (n=200) showed a mean total cell concentration of 21 million cells/ml sized 1-6 µm isolated from 10ml of whole blood after going through the SBSC protocol (p=0.0001) (Figure 3).
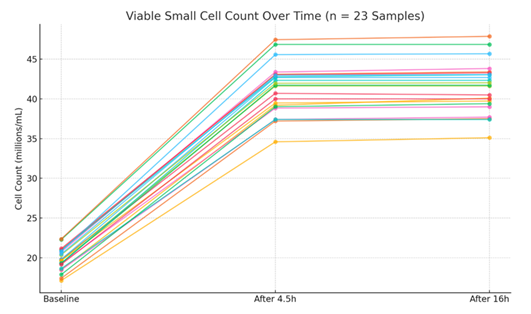
In the second stage, LunaStem™ analysis showed that the total viable cell concentration increased from ~20 million/ml to ~40 million/ml after 4.5h in cold storage, most prominently in the 1–3 µm size range. In multiple independent experiments (n=23), a consistent ~2–3-fold increase in viable small cells (~1 µm) was observed. The initial increase reached a plateau, with only marginal further growth during the following 12 hours. The mean increase was statistically significant (p=0.0001, one-way ANOVA). The fold increase showed similarity with a mean change of 2.09 ± 0.06 at 4.5h and 2.10 ± 0.06 at 16h. TOST equivalence testing with a margin of 0.1 (chosen as <5% relative fold change is biologically negligible) confirmed similarity between 4.5h and 16h fold increases (p < 0.001), indicating stabilization post-plateau (Figure 4). Yields ranged from 200–500 million SPSCs from 60 ml whole blood. Post-hoc power analysis for this ANOVA, assuming a large effect size (Cohen's f=0.4), yielded ~84% power (n=23, alpha=0.05), confirming the sample size was adequate to detect the observed large changes in cell counts (Table 1).
|
Sample |
Baseline (millions/mL) |
After 4.5h (millions/mL) |
After 16h (millions/mL) |
Fold Increase (4.5h) |
Fold Increase (16h) |
|
No. 1 |
1.85 × 107 |
3.92 × 107 |
4.01 × 107 |
2.12 |
2.17 |
|
No. 2 |
2.10 × 107 |
4.31 × 107 |
4.34 × 107 |
2.05 |
2.07 |
|
No. 3 |
1.98 × 107 |
4.07 × 107 |
4.05 × 107 |
2.06 |
2.05 |
|
No. 4 |
2.07 × 107 |
4.40 × 107 |
4.38 × 107 |
2.09 |
2.11 |
|
No. 5 |
1.97 × 107 |
4.27 × 107 |
4.27 × 107 |
2.16 |
2.16 |
|
No. 6 |
2.09 × 107 |
4.28 × 107 |
4.30 × 107 |
2.04 |
2.05 |
|
No. 7 |
2.23 × 107 |
4.68 × 107 |
4.68 × 107 |
2.10 |
2.10 |
|
No. 8 |
1.96 × 107 |
4.16 × 107 |
4.16 × 107 |
2.12 |
2.12 |
|
No. 9 |
1.96 × 107 |
3.95 × 107 |
3.97 × 107 |
2.01 |
2.02 |
|
No. 10 |
2.24 × 107 |
4.75 × 107 |
4.79 × 107 |
2.12 |
2.14 |
|
No. 11 |
2.12 × 107 |
4.30 × 107 |
4.33 × 107 |
2.03 |
2.05 |
|
No. 12 |
1.93 × 107 |
3.88 × 107 |
3.90 × 107 |
2.01 |
2.02 |
|
No. 13 |
2.08 × 107 |
4.56 × 107 |
4.57 × 107 |
2.19 |
2.20 |
|
No. 14 |
1.93 × 107 |
4.23 × 107 |
4.23 × 107 |
2.19 |
2.19 |
|
No. 15 |
1.92 × 107 |
4.17 × 107 |
4.17 × 107 |
2.16 |
2.16 |
|
No. 16 |
2.04 × 107 |
4.19 × 107 |
4.20 × 107 |
2.06 |
2.06 |
|
No. 17 |
1.71 × 107 |
3.46 × 107 |
3.51 × 107 |
2.02 |
2.05 |
|
No. 18 |
1.74 × 107 |
3.72 × 107 |
3.75 × 107 |
2.14 |
2.15 |
|
No. 19 |
1.92 × 107 |
4.00 × 107 |
4.01 × 107 |
2.09 |
2.09 |
|
No. 20 |
1.91 × 107 |
3.74 × 107 |
3.77 × 107 |
2.02 |
2.04 |
|
No. 21 |
1.90 × 107 |
4.29 × 107 |
4.30 × 107 |
2.10 |
2.10 |
|
No. 22 |
1.82 × 107 |
3.74 × 107 |
3.74 × 107 |
2.01 |
2.01 |
|
No. 23 |
1.96 × 107 |
3.90 × 107 |
3.94 × 107 |
2.18 |
2.20 |
Table 1: The changes of cell concentration between baseline, 4.5h in 4°C suspension and 16 hours in 4°C suspension. Calculated means and SD from individual samples confirm a consistent increase (baseline mean: 1.98 ± 0.15 × 107 cells/ml; after 4.5h: 4.14 ± 0.35 × 107 cells/ml), with low variability indicating reproducibility.
3.2 Viability and size distribution
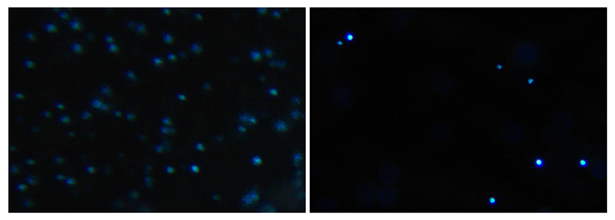
AO/PI staining revealed high cell viability in the 1–5 µm fraction, with over 90% of counted cells excluding PI after 4.5h of cold storage (viable/live cells not taking up PI nucleus stain). Automated sizing using the LunaStem™ confirmed that a majority of viable cells fell in the 1–3 µm range.
3.3 Nuclear and marker staining
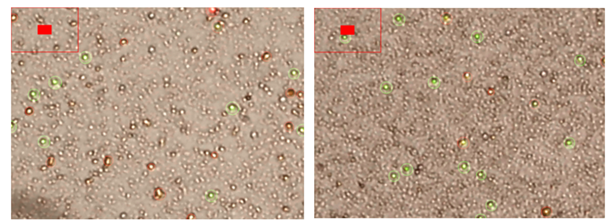
A validation step was conducted on a subset of 10 representative samples using both DAPI and Hoechst 33342 nuclear stains. This was done to verify the nuclear content of the smallest cells (≈1 µm), which were the ones that increased most prominently after cold storage. Although the LunaStem™ cell counter identified these cells as viable and within the expected size range, it did not register nuclear material in this fraction. Our staining confirmed that the vast majority of these small cells did in fact contain compact, DAPI- and Hoechst-positive nuclei, demonstrating that they are not apoptotic bodies or debris but represent viable, nucleated stem-like cells (Figure 5).
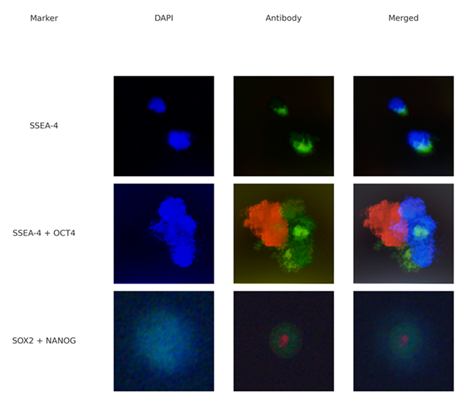
DAPI and Hoechst staining confirmed intact nuclear material in nearly all small cells. In the next analysis, immunostaining for pluripotency markers showed strong expression of: SSEA-4: Membrane-bound, green signal, OCT4: Nuclear, red fluorescence, SOX2 & NANOG: Co-localized nuclear signals in selected cells.
This panel shows immunostaining of small blood-derived stem cells (likely VSELs or SBSCs) using the markers SSEA-4, OCT4, SOX2 and NANOG. Each row represents a single cell or cell group stained with DAPI (blue, nuclei), antibodies (green = SSEA-4 or SOX2, red = OCT4 or NANOG), and a merged image combining all visible fluorescence channels (Figure 6; Table 2).
Panel 1: Immunofluorescence staining of human blood-derived cells. Top row: SSEA-4 expression (green). Second row: SSEA-4 and OCT4 co-expression (green + red). Third row: SOX2 and NANOG co-expression (green + red). DAPI stains nuclei in blue. Scale: ~1-5 µm.
|
Marker |
Localization |
Observation Notes |
|
SSEA-4 |
Membrane |
Strong green fluorescence in small cells (1–3 µm) |
|
OCT4 |
Nucleus |
Strong red nuclear signal, co-localized with DAPI and SSEA-4. |
|
SOX2 |
Nucleus |
Weaker intensity, nuclear localized but still present after 48h. |
|
NANOG |
Nucleus |
Nuclear staining shows a nuclear staining pattern filling a large portion of the cell, although a weaker signal still being present after 48h. |
|
DAPI |
Nucleus |
Uniform blue staining of condensed nuclei in all samples. |
Table 2: Summary of Immunofluorescence Marker Expression (n=10).
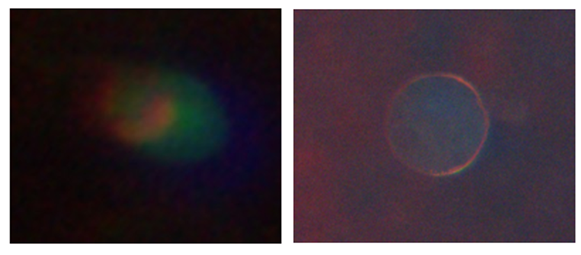
Representative cells after 48 hours. OCT4 and SSEA-4 expressions remain clearly visible (left picture). SOX2 and NANOG are still detectable but show weaker fluorescence signal intensity, however this configuration makes it easier to visibly see the cell membrane and shape (right picture). Nuclear morphology remains compact and small, below ~5µm.
4. Discussion
4.1 Biological Significance
This study provides compelling evidence that small pluripotent stem cells (SPSCs), isolated from peripheral blood, constitute a viable, nucleated, and pluripotent-like cell population. Their marked increase in concentration following 4.5h of cold storage, alongside expression of key pluripotency markers (OCT4, SOX2, NANOG, and SSEA-4), strongly supports the concept of a quiescent but inducible stem cell reservoir in adult peripheral blood [27,28]. Unlike apoptotic debris or vesicles, the smallest cells (1–3 µm) demonstrated intact nuclei via both DAPI and Hoechst staining and excluded PI, confirming their viability and structural integrity. This finding enhances the plausibility that these cells, previously described under categories such as VSELs and SBSCs, represent a functional stem cell compartment with developmental potential [29,30].
4.2 Comparison to Existing Literature
Our observations are consistent with earlier descriptions of VSELs by Ratajczak et al. [22,27,30] and more recent SBSC data from Filidou et al. [20], both of which highlight the small size, nuclear compactness, and pluripotency marker expression of these rare cells. However, this study builds upon prior work by incorporating real-time viability quantification (LunaStem™), cold-induced mobilization effects, and co-staining validation with two independent nuclear dyes. In contrast to mesenchymal stem cells (MSCs), which typically measure >15 µm and exhibit only multipotency [8], SPSCs combine minimal size with high lineage potential and nuclear stability. Previous concerns that small-sized cells might represent fragments, or non-viable entities are addressed here with clear visual and staining evidence. Additional support comes from studies on Muse cells [19,31] and mobilization under stress [32].
4.3 Clinical and Regulatory Implications
One of the most critical translational advantages of SPSCs is their compatibility with systemic administration. Unlike MSCs, which are known to become mechanically trapped in lung capillaries due to their large size ("pulmonary first-pass effect") [1-3], SPSCs, ranging from 1 to 5 µm, can likely bypass pulmonary filtration and reach systemic targets [21]. This could significantly enhance therapeutic reach, particularly for inflammatory, autoimmune, or degenerative diseases where a broader biodistribution is desirable. Furthermore, since these cells are isolated without enzymatic digestion, expansion, or genetic manipulation, they meet both EU/EMA and FDA criteria for minimal manipulation [4]. This regulatory status opens the door for autologous use under existing frameworks without requiring classification as Advanced Therapy Medicinal Products (ATMPs), the minimal manipulation also makes clinical translation more feasible and ethically sustainable [5,6].
Taken together, the biological characterization, literature consistency, and favorable regulatory profile support SPSCs as a highly promising candidate for further preclinical and clinical evaluation.
4.4 Potential Mechanisms of Cold-Induced Cell Enrichment
The observed 2-3-fold increase in viable small cells (1-3 µm) after 4.5h of cold storage at 4°C, with a subsequent plateau, suggests activation or mobilization of quiescent stem cells rather than rapid proliferation, consistent with the known quiescence of VSEL- and SBSC-like populations [22-30]. Cold storage in vacuum-sealed tubes without agitation likely induces mild hypoxia or metabolic stress, creating a low-oxygen microenvironment that mimics physiological injury responses [33,34]. This could trigger the release of chemokines such as stromal cell-derived factor-1 (SDF-1), hepatocyte growth factor (HGF), and leukemia inhibitory factor (LIF), which are known to mobilize VSELs from bone marrow or peripheral blood niches into circulation [33]. Studies on intermittent hypoxia in mice have shown similar mobilization of VSELs to peripheral blood, accompanied by activation of developmental transcriptional programs for repair and angiogenesis [34]. In our new protocol presented in this study, this stress-induced mobilization would explain the statistically significant increase (p=0.0001) and high viability (>90% AO/PI-negative), while the plateau after 4.5h may reflect exhaustion of the recruitable pool or stabilization under sustained cold conditions. However, suboptimal temperatures below 4°C could risk cellular damage, such as microtubule disruption, as reported in pluripotent stem cell suspensions [35]. These findings address concerns that the increased cells might be debris, as confirmed by intact nuclei (DAPI/Hoechst-positive). Overall, this novel cold-enrichment method appears to harness hypoxia-like signals for efficient SPSC isolation, warranting further exploration of oxygen dynamics in future optimizations.
4.5 Broader Implications for Regenerative Medicine
The high yields of SPSCs (200-500 million from 60 ml blood) and their pluripotent-like marker expression (OCT4, SOX2, NANOG, SSEA-4) position them as a scalable, autologous alternative to embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), with inherently lower tumorigenicity risks due to their adult origin and lack of genetic manipulation [16,17]. Recent reviews underscore pluripotent/multipotent stem cells in peripheral blood as "underutilized reservoirs" for regenerative therapies, particularly in inflammatory or degenerative conditions where systemic biodistribution is essential [36]. The novel protocol's minimal manipulation aligns with regulatory frameworks [4-6], potentially enabling rapid translation to clinical applications like autoimmune diseases, chronic pain, or anti-aging interventions, where small cells could evade pulmonary entrapment and target distant sites [1-3,21]. The limitations of this study, such as the absence of functional differentiation assays, are mitigated by the robust viability and marker data presented, which provide a foundation for in vivo studies. The findings presented herein align with ongoing clinical trials of human pluripotent stem cell-derived therapies showing safety and efficacy in similar contexts [37]. Thus, SPSCs offer a promising bridge between basic research and clinic, emphasizing the need for collaborative trials to validate their regenerative potential (Table 3).
|
Attribute |
SPSCs (This Study) |
VSELs |
SBSCS |
Muse Cells |
|
Size |
<5 µm (1–3 µm prominent) |
2–5 µm (mice); 5–7 µm (humans) |
~3–7 µm (similar to VSELs) |
10–15 µm (larger than VSELs) |
|
Key Markers |
OCT4, NANOG, SOX2, SSEA-4; DAPI/Hoechst-positive |
OCT4, NANOG, SSEA-1/4, CXCR4, CD133; Lin-, CD45- |
OCT4, NANOG, SOX2, SSEA-4; similar to VSELs |
SSEA-3, CD105 (BM); CD45 (PB); pluripotent genes like OCT4 |
|
Location/Source |
Peripheral blood (human) |
Bone marrow, PB, solid organs |
Peripheral blood, bone marrow |
Bone marrow, PB, connective tissues |
|
Mobilization Triggers |
Cold storage (mild hypoxia/stress); rapid 2–3-fold increase |
Hypoxia, injury, stress (via SDF-1/CXCR4, HGF, LIF) |
Stress, hypoxia (similar to VSELs) |
Stress, injury (via S1P–S1PR2 axis) |
|
Differentiation Potential |
Pluripotent-like (markers suggest trilineage) |
Trilineage (ecto-, meso-, endoderm); cardiac/endothelial |
Trilineage; regenerative in blood-derived models |
Trilineage; spontaneous differentiation in vivo |
|
Unique Features |
High yields (200–500M from 60 ml); minimal manipulation |
Quiescent; mobilized in MI/stroke; no teratomas |
Blood-derived; easy isolation via centrifugation |
Stress-enduring; bystander effects; no HLA matching needed |
Table 3: Comparative Overview of SPSCs and Related Pluripotent-Like Stem Cell Populations.
Table 3, This comparison highlights shared characteristics among SPSCs and established pluripotent-like populations, such as small size and core markers (e.g., OCT4, NANOG), while emphasizing SPSCs' unique cold-induced mobilization from peripheral blood. Unlike larger Muse cells [25,31], SPSCs' <5 µm diameter supports potential systemic delivery without pulmonary entrapment. These similarities underscore SPSCs as a hybrid VSEL/SBSC-like entity, with advantages in minimal manipulation and high yields for regenerative applications [16,18,20]. Future studies could quantify differentiation efficiencies across these groups.
5. Conclusion
In conclusion, this novel cold-enrichment method yields high numbers of pluripotent-like SPSCs from peripheral blood, with evidence of a 2-fold increase (p=0.0001) and regulatory compliance, paving the way for autologous regenerative therapies. The protocol's minimal manipulation and high viability position SPSCs as a safe, scalable alternative to traditional stem cell sources, potentially addressing limitations in biodistribution and tumorigenicity.
Future directions include in vivo studies to assess differentiation. Additionally, optimizing oxygen levels during cold storage could further enhance yields, while early-phase clinical trials in conditions like chronic pain or autoimmune disorders would validate therapeutic efficacy. Overall, these advancements could accelerate the adoption of SPSCs in personalized medicine.
6. Declarations
Ethical Considerations
All blood donors provided informed consent before donating blood samples, all data collected was anonymous.
Ethics Approval
According to the Ethics Commission of Stockholm, Sweden Ethical review was not required for this preclinical laboratory analysis using anonymized samples from healthy adult volunteers and patient informed consent whenever the data were acquired, saved and treated anonymously.
The study was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All Blood donors provided informed consent, no individual data was included in the manuscript.
Consent for Publication
This manuscript does not contain any individual person’s data. All data exposed in this manuscript was anonymized.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgements, Author Contributions
The first author TO, was the main contributor to the manuscript, data collection and sample analysis was also performed by the second author AR, besides complementary writing, review and editing.
All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Authors’ contributions
All texts, design, literature review and drafting of this study were done by TO and AR, responsible for the submitted manuscript.
Availability of data and materials
All data generated or analyzed during this study can be provided by the corresponding authors upon reasonable request and is available for review by the Editor-in-Chief of this journal.
References
- Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells and Development 18 (2009): 683-692.
- Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: The lung barrier. Transplant Proceedings 39 (2007): 573-576.
- Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Stem Cells 30 (2012): 1175-1177.
- European Medicines Agency (EMA). Reflection paper on classification of advanced therapy medicinal products (2015). Retrieved from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-classification-advanced-therapy-medicinal-products_en.pdf-0
- S. Food and Drug Administration (FDA). Regulatory considerations for human cells, tissues, and cellular and tissue-based products: Minimal manipulation and homologous use (2017). Retrieved from: https://www.fda.gov/media/109176/download
- Lysaght T, Kerridge IH, Sipp D, et al. Ethical and Regulatory Challenges with Autologous Adult Stem Cells: A Comparative Review of International Regulations. Bioethical Inquiry 14 (2017): 261-273.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 (2006): 663-676.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 284 (1999): 143-147.
- Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nature Biotechnology 27 (2009): 743-745.
- Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Advances in Cancer Research 100 (2008): 133-158.
- Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 474 (2011): 212-215.
- Bulic-Jakus F, Ulamec M, Vlahovic M, et al. Of mice and men: teratomas and teratocarcinomas. Coll Antropol 30 (2006): 921-4.
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136 (2009): 701-713.
- Zhong C, Liu M, Pan X, et al. Tumorigenicity risk of iPSCs in vivo: nip it in the bud. Precis Clin Med 5 (2022): pbac004.
- Itakura G, Kawabata S, Ando M, et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Reports 8 (2017): 673-684.
- Wakao S, Kitada M, Kuroda Y, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proceedings of the National Academy of Sciences 108 (2011): 9875-9880.
- Fisch SC, Gimeno ML, Phan JD, et al. Pluripotent nontumorigenic multilineage differentiating stress enduring cells (Muse cells): a seven-year retrospective. Stem Cell Res Ther 8 (2017): 227.
- Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4+ SSEA-1+ Oct-4+ stem cells identified in adult bone marrow. Leukemia 20 (2006): 857-869.
- Tatsumi K, Kushida Y, Wakao S, et al. Protocols for Isolation and Evaluation of Muse Cells. Adv Exp Med Biol 1103 (2018): 69-101.
- Filidou E, Kandilogiannakis L, Tarapatzi G, et al. A Simplified and Effective Approach for the Isolation of Small Pluripotent Stem Cells Derived from Human Peripheral Blood. Biomedicines 11 (2023): 787.
- Aprile D, Patrone D, Peluso G, et al. Multipotent/pluripotent stem cell populations in stromal tissues and peripheral blood: exploring diversity, potential, and therapeutic applications. Stem Cell Res Ther 15 (2024): 139.
- Ratajczak MZ, Ratajczak J, Kucia M. Very Small Embryonic-Like Stem Cells (VSELs): An Update and Future Directions. Circ Res 124 (2019): 208-210.
- Bhartiya D, Jha N, Tripathi A, et al. Very small embryonic-like stem cells have the potential to win the three-front war on tissue damage, cancer, and aging. Front. Cell Dev Biol 10 (2023): 1061022.
- D’Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells. Journal of Cell Science 117 (2004): 2971-81.
- Kuroda Y, Wakao S, Kitada M, et al. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8 (2013): 1391-1415.
- Lahlil R, Scrofani M, Barbet R, et al. VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Reviews and Reports 14 (2018): 510-524.
- Ratajczak M, Zuba-Surma E, Wojakowski W, et al. Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia 28 (2014): 473-484.
- Bhartiya D, Shaikh A, Nagvenkar P, et al. Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev 21 (2012): 1-6.
- Thetchinamoorthy K, Jarczak J, Kieszek P, et al. Very small embryonic-like stem cells (VSELs) on the way for potential applications in regenerative medicine. Front. Bioeng. Biotechnol 13 (2025): 1564964.
- Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, et al. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol 36 (2008): 742-51.
- Kuroda Y, Dezawa M. Mesenchymal stem cells and their subpopulation, pluripotent muse cells, in basic research and regenerative medicine. Anat Rec (Hoboken) 297 (2014): 98-110.
- Wakao S, Kushida Y, Dezawa M. Basic Characteristics of Muse Cells. Adv Exp Med Biol 1103 (2018): 13-41.
- Gharib SA, Khalyfa A, Kucia MJ, et al. Transcriptional landscape of bone marrow-derived very small embryonic-like stem cells during hypoxia. Respir Res 12 (2011): 63.
- Guerin CL, Blandinières A, Planquette B, et al. Very Small Embryonic-like Stem Cells Are Mobilized in Human Peripheral Blood during Hypoxemic COPD Exacerbations and Pulmonary Hypertension. Stem Cell Rev Rep 13 (2017): 561-566.
- Kitahata S, Tanaka Y, Hori K, et al. Critical Functionality Effects from Storage Temperature on Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Suspensions. Sci Rep 9 (2019): 2891.
- Aprile D, Patrone D, Peluso G, et al. Multipotent/pluripotent stem cell populations in stromal tissues and peripheral blood: exploring diversity, potential, and therapeutic applications. Stem Cell Res Ther 15 (2024): 139.
- Kirkeby A, Main H, Carpenter M. Pluripotent stem-cell-derived therapies in clinical trial: A 2025 update. Cell Stem Cell 32 (2025): 10-37.
Related PubMed Articles
- Chemical reprogramming of human blood cells to pluripotent stem cells.
- Inducing mononuclear cells of patients with CADASIL to construct a CSVD disease model.
- Human stem cells with in vivo high plasticity generated by cell-cell communication.
- Next-generation sequencing protocol of hematopoietic stem cells (HSCs). Step-by-step overview and troubleshooting guide.
- Pericytes Enrich the Basement Membrane and Reduce Neutrophil Transmigration in an In Vitro Model of Peripheral Inflammation at the Blood-Brain Barrier.
- Generation of RAB4A homozygous knockout induced pluripotent stem cell (iPSC) line.
- A monoclonal antibody recognizing CD98 on human embryonic stem cells shows anti-tumor activity in hepatocellular carcinoma xenografts.
- Establishment of a novel amyotrophic lateral sclerosis patient (TARDBP (N345K/+))-derived brain microvascular endothelial cell model reveals defective Wnt/β-catenin signaling: investigating diffusion barrier dysfunction and immune cell interaction.
- Multipotent/pluripotent stem cell populations in stromal tissues and peripheral blood: exploring diversity, potential, and therapeutic applications.
- SET domain containing 2 promotes megakaryocyte polyploidization and platelet generation through methylation of α-tubulin.