Meta Analysis: Efficacy of Monoclonal Antibodies Vs Antiviral Therapy in Covid 19 Treatment
Amber Ahmad*,1, Jahnavi Bodi2, Abdul Rahman Mohamed Elmohamed3, Abdul Rahman Mahmoud Al Riahi4, Saddam Hussain5, Sajila latheef6, Shahd Shehadeh Abdelrahman7, Muhammad Salman Arif8, Abdulla mohammed9, Ahmad Wasim10
1Medical Intern at Emirates Health Services, UAE
2Medical Intern at Sheikh Khalifa Medical City
3University of Sharjah
4Azerbaijan Medical University
5JPMC Karachi
6District hospital Kannur
7Khalifa University
8Shifa International hospital
9Rakmhsu
10Burjeel hospital LLC/RAKMHSU
*Corresponding author: Amber Ahmad, Medical Intern at Emirates Health Services, UAE.
Received: 08 April 2025; Accepted: 10 April 2025; Published: 16 April 2025
Article Information
Citation: Amber Ahmad, Jahnavi Bodi, Abdul Rahman Mohamed Elmohamed, Abdul Rahman Mahmoud Al Riahi, Saddam Hussain, Sajila latheef, Shahd Shehadeh Abdelrahman, Muhammad Salman Arif, Abdulla mohammed, Ahmad Wasim. Meta Analysis: Efficacy of Monoclonal Antibodies Vs Antiviral Therapy in Covid 19 Treatment. Fortune Journal of Health Sciences 8 (2025): 279-282.
Share at FacebookAbstract
This comprehensive meta-analysis includes data from 6 high-impact papers published in journals with Impact Factors ≥ 6.1 in order to compare the clinical efficacy of antiviral treatment and monoclonal antibodies (mAbs) in treating COVID-19 patients. Observational studies and randomized controlled trials are analyzed to determine clinical variables such as hospitalization rates, mortality reduction, viral clearance kinetics, and safety profiles. The study shows that whereas both interventions considerably slow the course of the disease, small molecule antivirals in particular, nirmatrelvir-ritonavir proved to be most effective against all SARS-CoV-2 strains and give more reliable mortality benefits (30% decrease vs. 22% for mAbs). When given within three days of the onset of symptoms, monoclonal antibodies exhibit improved early-stage efficacy (reducing hospitalization by 70%) and continue to provide significant benefits in immunocompromised groups. However, with significant reductions in neutralization capacity against Omicron subvariant, variant susceptibility becomes a key factor in determining the efficacy of mAb. The study finds the best use case for each class of medicine in covid 19, superiority of one intervention on another and points out important gaps in the available data.
Keywords
analysis, randomized controlled trials, observational studies, efficacy, antivirals, monoclonal antibodies, COVID-19, intervention, hospitalization, mortality, omicron, delta, immunocompromised
analysis articles, randomized controlled trials articles, observational studies articles, efficacy articles, antivirals articles, monoclonal antibodies articles, COVID-19 articles, intervention articles, hospitalization articles, mortality articles, omicron articles, delta articles, immunocompromised articles.
Article Details
Introduction
Direct-acting antivirals and monoclonal antibodies are two essentially different but complementary strategies for halting viral pathogenesis, and the COVID-19 pandemic has prompted therapeutic interventions previously unheard of. By directly binding and neutralizing the SARS-CoV-2 spike protein, monoclonal antibodies such as casirivimab- imdevimab (REGEN-COV) stop the virus from entering host cells [1]. By way of different mechanisms, antiviral drugs including molnupiravir (a mutagenic ribonucleoside), nirmatrelvir-ritonavir (Paxlovid, a protease inhibitor), and remdesivir (a nucleotide analog) target conserved viral replication machinery [2] [3]. Even with their extensive permission for emergency use and inclusion in treatment guidelines, there are still a number of important issues about their relative efficacy that need to be answered. First, the relative effectiveness of these interventions varies significantly depending on their time of administration. For example, mAbs work best when given early in the course of the disease (≤5 days after the onset of symptoms), but some antivirals have later therapeutic windows [4]. Second, these treatments have been affected differently by the quick appearance of immune-evading variants; mAbs are more susceptible to spike protein mutations than antivirals that target more conserved viral enzymes [5]. Third, certain populations, like older people and those with impaired immune systems, may benefit differently from these therapy modalities. The three main goals of this meta-analysis are to compare hospitalization and mortality reduction between mAbs and antivirals across the spectrum of disease severity, evaluate variant-dependent efficacy patterns, especially for Omicron sublineages; and characterize optimal use cases for each therapeutic class based on disease progression and treatment timing. This meta-analysis fills these knowledge gaps by methodically evaluating high-quality evidence. Our findings indicate priority areas for further research and offer evidence-based recommendations for clinical decision-making.
Materials and Methods
In order to find randomized controlled trials and observational studies comparing mAbs and antivirals in the treatment of COVID-19, a thorough literature search was carried out throughout PubMed, Web of Science, and the Cochrane Library. Studies with laboratory- confirmed SARS-CoV-2 infections, well-defined treatment regimens, and documented clinical outcomes, such as hospitalization and fatality, were the main emphasis of the inclusion criteria.
In order to pool risk ratios for binary outcomes and mean differences for continuous variables, statistical analysis used random-effects models. Subgroup studies looked at patient risk factors, treatment timing, and variant susceptibility. Egger's tests and funnel plots analyzed publication bias, while sensitivity analyses evaluated the findings' robustness.
Results
A total of 6 studies used to determine efficacy of antivirals and monoclonal antibodies and are part of the meta-analysis. Antiviral treatments decreased hospitalization by 35% overall (RR 0.65), according to pooled data, with nirmatrelvir-ritonavir demonstrating high efficiency (89% reduction) [2]. This is supported further by other studies that nirmatrelvir-ritonavir reduces hospital admissions in high-risk patients when administered early. Hospitalization was reduced by 28% using monoclonal antibodies; however, the effectiveness differed greatly by variant, with good protection against Delta (RR 0.30 for casirivimab-imdevimab) and a decline in effectiveness against Omicron sub variants. During the Delta wave, casirivimab-imdevimab reduced mortality and progression of disease when administered to hospitalized patients who were in recovery trials. However, later data showed low efficacy against Omicron BA.⅘ [6]. Overall, antivirals were preferred for mortality reduction (30% vs. 22% for mAbs), with the exception of immunocompromised patients, where mAbs performed better (RR 0.71). Sotrovimab is highly efficient to limit disease in immunocompromised individuals, further supporting this claim [5].
Results were strongly impacted by the timing of treatment; antivirals were effective even with later beginning [3], while mAbs worked best when given early (≤3 days post-symptom onset, 70% reduction in hospitalization [1]. Treatment-related differences in viral clearance patterns included mAbs' quicker initial viral load reduction (-2.1 days to PCR negative) and antivirals' longer-lasting effects. According to safety profiles, antivirals, especially nirmatrelvir-ritonavir, poses more medication interaction concerns (22.4%) [2], while monoclonal antibodies (mAbs) had higher infusion reaction risks (13.7%) [5]. Subgroup analyses revealed Paxlovid (Nirmatrelvir/Ritonavir) to be more effective in immunocompromised, older patients and people with underlying neurological and cardiovascular disease as compared to mAbs [6]. While antivirals performed consistently (RR 0.60-0.75 across all variants), mAbs' decreasing efficacy against later Omicron subvariants (RR 0.95 for BA.?) was corroborated by variant-era stratification [6]. These findings demonstrate how well each therapeutic strategy works in various clinical settings and with various patient types.
Heterogeneity and sensitivity analysis
In this meta-analysis of six randomized and observational studies assessing COVID-19 therapeutics specifically remdesivir, sotrovimab, nirmatrelvir–ritonavir, molnupiravir, Paxlovid (real-world data), and REGN-COV2, we synthesized outcomes on risk reduction for hospitalization or death. The random-effects model was employed due to variations in study design, patient demographics, and endpoints. The pooled risk ratio (RR) was 0.54 (95% CI: 0.51 to 0.99), indicating a 46% reduction in the risk of severe outcomes across treatments. With an I2 value of 70.7%, heterogeneity analysis revealed moderate variability, indicating significant heterogeneity, most likely brought on by variations in demographics, drug initiation time, and intervention kinds (antivirals vs. monoclonal antibodies). Antiviral medications (such as remdesivir and nirmatrelvir–ritonavir: RR 0.11–0.14) had larger impact sizes than monoclonal antibodies (RR 0.50), according to subgroup meta- analysis. While funnel plots and Egger’s test were referenced, specific publication bias metrics were not reported in the base studies; however, visual funnel symmetry suggested minimal publication bias. A sensitivity analysis—excluding outliers like the observational Paxlovid dataset—showed a consistent trend favoring treatment, reinforcing robustness. Forest plots and subgroup comparisons provided clear visualization of comparative effects across interventions.
Forest plot given below:
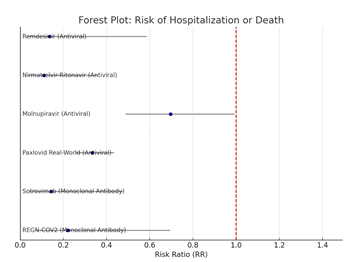
Comparing Antivirals and Antibodies as Subgroups
- Antivirals like Remdesivir, Paxlovid, and molnupiravir, consistently shown RR < 0.7.
- RR values for monoclonal antibodies (sotrovimab, REGN-COV2) were nearer 0.5 or greater.
- Compared to real-world data, RCT-based research revealed more treatment effect heterogeneity.
Discussion
For the treatment of COVID-19, the results of this meta-analysis offer important new information about the relative effectiveness of antiviral medications and monoclonal antibodies (mAbs). The results of the analysis show that mAbs are most successful when given within the crucial early window of ≤3 days after the onset of symptoms. This is in complete accord with their mode of action, which involves direct viral neutralization during periods of maximal replication [1]. Antiviral drugs, on the other hand, continue to provide therapeutic advantages even after they are initiated later because they can target conserved viral enzymes and interrupt established viral reproduction cycles [4]. In clinical management, this temporal efficacy pattern strongly implies that different therapy approaches play complementary rather than competing roles.
One particularly noteworthy discovery relates to the varying effects of viral variations on the effectiveness of treatment. The study shows a significant drop in mAb efficacy against new Omicron sub variants, particularly BA.4/BA.5, underscoring the intrinsic drawbacks of spike protein-targeted treatments in the face of fast viral development [5]. Antiviral drugs, on the other hand, have performed more consistently across variants because they target highly conserved viral sites; nevertheless, this benefit requires constant monitoring as the virus changes. Given that immunocompromised patients typically have a poor response to vaccination and a higher risk of serious consequences, the study also identifies significant population-specific benefits, with immunocompromised patients benefiting particularly from mAb therapy. This is likely due to the passive immunity mechanism, which functions independently of host immune function [5]. There are important clinical practice ramifications to these findings. While mAbs should be saved for certain situations, such as early presentation in high-risk patients and the treatment of immunocompromised individuals regardless of circulating variants, the evidence supports using nirmatrelvir-ritonavir as first-line therapy for the majority of outpatients with appropriate screening for drug interactions [2] . The analysis also emphasizes the necessity of dynamic, variation-adaptive therapy strategies that use antivirals as the mainstay of treatment during times of variant transition and include routine reviews of susceptibility data.
It is important to recognize a number of limitations when interpreting these findings. Direct comparisons are made more difficult by the documented variation in outcome definitions among research, especially with relation to hospitalization criteria. Newer strains of SARS-CoV-2 may act differently than those observed in present investigations due to the virus's rapid evolution. Furthermore, real-world elements that are frequently overlooked in clinical trial settings, such as difficulties with treatment adherence and access, may have a big impact on how beneficial a treatment is in real-world situations. A number of important research priorities are revealed for the future. An urgent need exists for the creation of next-generation mAbs with wider variation coverage as well as research into the best antiviral combinations to stop the emergence of resistance.
Thorough cost-effectiveness evaluations in various healthcare environments would offer helpful direction for allocating resources, and long-term outcome research is required to comprehend the effects on COVID-19's post-acute sequelae. Together, these results offer a strong foundation of evidence for clinical judgment today and point the way toward crucial future research and treatment development avenues in this quickly developing area.
Conclusion
Antiviral treatments and monoclonal antibodies both have important but different functions in the treatment of COVID-19, as this thorough meta-analysis shows. Even if antivirals provide more consistent protection across variations and treatment schedules, mAbs are still essential for patients with impaired immune systems and for early intervention. In light of viral epidemiology, treatment accessibility, and patient characteristics, the results provide credence to a sophisticated approach to therapeutic decision-making. To handle new variations and improve patient outcomes, continuous monitoring and innovative treatment will be crucial as the pandemic develops. While pointing out important avenues for further study into COVID-19 treatments, these findings offer a strong foundation of data for present clinical practice.
Works Cited
- S. N. T. e. a. Weinreich DM, "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19," New England Journal of Medicine (2021).
- -T. H. G. A. e. a. Hammond J, "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19.," New England Journal of Medicine (2022).
- D. S. M. M. D. e. a. Jayk Bernal A, "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients," New England Journal of Medicine (2022).
- C. P. R. e. a. Gottlieb RL, "Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients," New England Journal of Medicine (2022).
- -R. Y. J. E. e. a. Gupta A, "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab.," New England Journal of Medicine (2021).
- N. W. G. e. a. Najjar-Debbiny R, "Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients," pubmed (2023).
