Meta-Analysis: The Impact of Heart Failure Medications in Improving Left Ventricular Function in Chemotherapy Induced Cardiomyopathy
Azzah Muhammad Asharaf*,1, Mariyam Ansari2, Lamis Elfateh Mohamed Ali3, Yasmeen sharif Mahmoud4, Ahmed Ibrahim haji Ahmed5, Muneeb Mazhar6, Saif Khalid7, Hamda hamad alefari8, Sara El Moussa9, Ahmad Wajdi M.Ali10
1Gulf Medical University
2Liaquat college of medicine and dentistry
3University of Medical Sciences and Technology
4The University of Georgia
5Ras Al Khaima Medical & Health Science university
6Ras al khaima medical and health sciences university
7Royal College of Surgeons Ireland
8United Arab Emirates University
9Khalifa University
10University of sharjah
*Corresponding author: Azzah Muhammad Asharaf. Gulf Medical University, UAE.
Received: 22 July 2025; Accepted: 31 July 2025; Published: 12 August 2025
Article Information
Citation: Azzah Muhammad Asharaf, Mariyam Ansari, Lamis Elfateh Mohamed Ali, Yasmeen sharif Mahmoud, Ahmed Ibrahim haji Ahmed, Muneeb Mazhar, Saif Khalid, Hamda hamad alefari, Sara El Moussa, Ahmad Wajdi M.Ali. Meta-Analysis: The Impact of Heart Failure Medications in Improving Left Ventricular Function in Chemotherapy Induced Cardiomyopathy. Cardiology and Cardiovascular Medicine 9 (2025): 308-314.
Share at FacebookAbstract
Background: One major side effect of cancer treatment is chemotherapy induced cardiomyopathy (CIC), especially from anthracyclines and HER2-targeted drugs. Early administration of heart failure drugs such as beta-blockers (BBs), angiotensin receptor blockers (ARBs), statins, mineralocorticoid receptor antagonists (MRAs), ACE inhibitors (ACEIs) and more recent drugs like angiotensin receptor–neprilysin inhibitors (ARNIs) has demonstrated promising effects in reducing cardiac damage. Major cardiology societies like the American Heart Association (AHA), European Society of Cardiology (ESC), and American College of Cardiology (ACC) recommend these agents as the cornerstone of Guideline- Directed Medical Therapy (GDMT), which is the standard approach for treating heart failure and is being used to stop or reverse cardiac dysfunction in patients who have cardiomyopathy caused by cancer treatment.
Objective: The objective of this study is to assess and compile the available data regarding the effectiveness of heart failure drugs in maintaining or improving left ventricular ejection fraction (LVEF) in patients suffering from cardiomyopathy caused by chemotherapy.
Methods: This study includes peer reviewed studies which mainly are expert reviews, cohort studies, meta-analysis and randomized controlled trials (RCTs). Reports which focused on evaluating the impact of heart failure drugs on LVEF in populations exposed to chemotherapy were included. Pooled effect sizes, if available, were used to synthesize the data, and the I2 statistic was used to assess heterogeneity. Risk ratios and standardized mean differences were extracted to measure treatment effects across drug classes.
Results: Among the best agents for improving left ventricular ejection fraction (LVEF) were enalapril (+7.6%, CI: 5.3–9.9%) and spironolactone (MD: +12.8%, CI: 7.9–17.7%) [1], [2]. In refractory cases, sacubitril/valsartan showed a high LVEF recovery [3]. Although ARBs showed mixed results, telmisartan stood out very effective because of its anti-inflammatory qualities [4]. The likelihood of LVEF recovery was considerably enhanced by early initiation of guideline directed medical therapy (GDMT) (OR = 9.39, p < 0.001) [5]. Heterogeneity was moderate to high (I² > 60%) and publication bias was minimal.
Conclusion: In patients with chemotherapy induced cardiomyopathy, heart failure medications, especially when started early, significantly improve or preserve LVEF. Preventive and therapeutic cardio-oncology protocols should give priority to GDMT, particularly ACEIs, MRAs, and ARNIs.
Keywords
Chemotherapy induced Cardiomyopathy (CIC), mineralocorticoid receptor antagonists (MRAs), angiotensin converting enzyme inhibitors (ACEIs), beta-blockers (BBs), angiotensin receptor–neprilysin inhibitors (ARNIs), statins, guideline-directed medical therapy (GDMT), left ventricular ejection fraction (LVEF), randomized controlled trials (RCTs), cohort study, heterogeneity analysis
Chemotherapy induced Cardiomyopathy (CIC) articles, mineralocorticoid receptor antagonists (MRAs) articles, angiotensin converting enzyme inhibitors (ACEIs) articles, beta-blockers (BBs) articles, angiotensin receptor?neprilysin inhibitors (ARNIs) articles, statins articles, guideline-directed medical therapy (GDMT) articles, left ventricular ejection fraction (LVEF) articles, randomized controlled trials (RCTs) articles, cohort study articles, heterogeneity analysis articles.
Article Details
Introduction
It has been repeatedly shown that anthracyclines, HER2 inhibitors, and other chemotherapeutic agents cause cardiomyocyte apoptosis, interstitial fibrosis and mitochondrial dysfunction, all of which lead to left ventricular systolic dysfunction [6, 7]. Chemotherapy induced cardiomyopathy (CIC), which frequently manifests as heart failure with reduced ejection fraction (HFrEF), is characterized by these pathophysiological alterations. In oncology care, routine cardiac surveillance is essential because CIC can be progressive and irreversible if not identified and treated early [5, 8]. Over the past 20 years, a growing corpus of research has addressed this clinical challenge by examining the potential of conventional heart failure medications to either delay the onset of CIC or aid in the recovery of left ventricular function after dysfunction has already occurred. Both RCTs and meta-analysis have shown that angiotensin-converting enzyme inhibitors (ACEIs), like enalapril and perindopril, are significantly effective in increasing left ventricular ejection fraction (LVEF), especially when started early in the course of myocardial injury [1, 8, 9]. In high-risk groups like those receiving anthracyclines or trastuzumab, beta-blockers like carvedilol and bisoprolol have demonstrated mild but reliable cardioprotective effects, particularly when used in conjunction with ACEIs [2, 10].
Because of their antifibrotic and anti-inflammatory qualities, mineralocorticoid receptor antagonists (MRAs), most notably spironolactone, have shown exceptional effectiveness in maintaining or restoring LVEF. Spironolactone has shown the largest effect size among all conventional heart failure therapies in the context of CIC, according to a number of trials and network meta-analysis [1, 2, 11]. Sacubitril/valsartan, an angiotensin receptor–neprilysin inhibitor (ARNI), has drawn attention recently due to its superior hemodynamic and neurohormonal modulation. In patients who are not responding to ACEIs and beta-blocker therapy, case reports and prospective cohort studies demonstrate remarkable recovery of LVEF, with benefits extending to reverse remodelling and biomarker reduction after usage of ARNIs [3], [12]. Though randomized trial data for ARNIs in this population are still limited, ongoing trials such as PRADA II still support its effectivity [13]. Angiotensin receptor blockers (ARBs) have not consistently improved LVEF in clinical studies. However, telmisartan, an ARB with partial PPAR-γ agonist activity, has demonstrated class-exception behavior in improving strain rate (SR) and minimizing oxidative stress and inflammation [2, 4, 14]. Statins, which are typically prescribed to treat dyslipidemia, have also shown increasing usefulness in this area. According to observational cohort data, they might increase the chance of LVEF recovery in CIC patients, possibly by having pleiotropic effects on inflammation and endothelial function [15]. Including them in cardioprotective regimens may provide an extra benefit, especially for patients who are older and at higher risk.
The need for early detection and customized initiation of heart failure therapies in cancer patients who are at risk of cardiotoxicity is highlighted by various studies. Timely intervention, ideally within the first few weeks of left ventricular decline, significantly increases the likelihood of full or partial myocardial recovery, as shown in longitudinal and multi-center cohorts [5], [8]. To improve treatment algorithms and incorporate new agents into cardio-oncology practice, more research through extensive and prospective trials is required.
Hence, this meta-analysis was carried out to systematically assess the relative effectiveness of heart failure medications in enhancing LVEF and reducing clinical deterioration in patients with chemotherapy-induced cardiomyopathy.
Methods
In this meta-analysis, evidences from peer-reviewed studies that looked at how heart failure drugs affect left ventricular function in patients undergoing cardiotoxic chemotherapy were combined. High quality review articles i.e., randomized controlled trials (RCTs), cohort studies, systematic or network meta-analysis, case reports and expert reviews which assessed human or animal populations exposed to known cardiotoxic chemotherapeutic agents, such as anthracyclines (e.g., doxorubicin, epirubicin), trastuzumab, cyclophosphamide, and cladribine and receiving concurrent treatment with guideline-directed heart failure pharmacotherapy were included. Drugs of interest were ACE inhibitors like enalapril and perindopril, angiotensin receptor blockers (ARBs) like telmisartan and candesartan, beta-blockers like carvedilol and bisoprolol, mineralocorticoid receptor antagonists (MRAs) like spironolactone, angiotensin receptor- neprilysin inhibitors (ARNIs) like sacubitril/valsartan, and statins. Cardiac functional outcomes were evaluated, particularly left ventricular ejection fraction (LVEF), global longitudinal strain (GLS), myocardial strain rate (SR) or cardiac biomarkers like NT-proBNP and troponin I . Every study had to be peer-reviewed and include data that could be extracted. Studies that did not use a specific intervention under study, had a non-cardiac focus or were opinion pieces, editorials, or commentaries without primary data were not included.
A random-effects model is employed to pool the data and take into consideration the wide variations in cancer types, treatments, and measurement techniques among the studies. Weighted mean differences (WMDs) were used for continuous outcomes like LVEF and standardized mean differences (SMDs) when the studies measured LVEF differently (for example, by using different imaging techniques). Particularly in long-term follow-up studies, risk ratios or odds ratios were used for outcomes like heart failure rates or recovery of LVEF to over 50%.
The I2 statistic was used to measure the degree of heterogeneity in the study results. Low heterogeneity was defined as an I2 value less than 25%, moderate heterogeneity as one between 25% and 75% and high heterogeneity as one greater than 75%. Due to variations in population age, baseline cardiovascular risk, medication initiation timing and cumulative chemotherapy dose, significant heterogeneity was anticipated and taken into consideration. To ascertain whether the directionality of findings remained robust, sensitivity analysis was conducted by eliminating studies that were considered to be at high risk of bias, such as isolated case reports or single-arm cohorts. Subgroup comparison was conducted by drug class and improvement in LVEF. As documented in studies like OVERCOME trial, analysis was further stratified by cancer type whenever feasible, with breast cancer and hematologic malignancies being the most common cancer types [9]. Numbers and results from large existing meta-analysis [1, 2] were combined with findings from clinical trials and observational studies to make sure robustness of results and multiple levels of strong evidence, following the best practices in cardiology research. Below is the Prisma flow chart (Figure 1) showing systematic screening of studies:
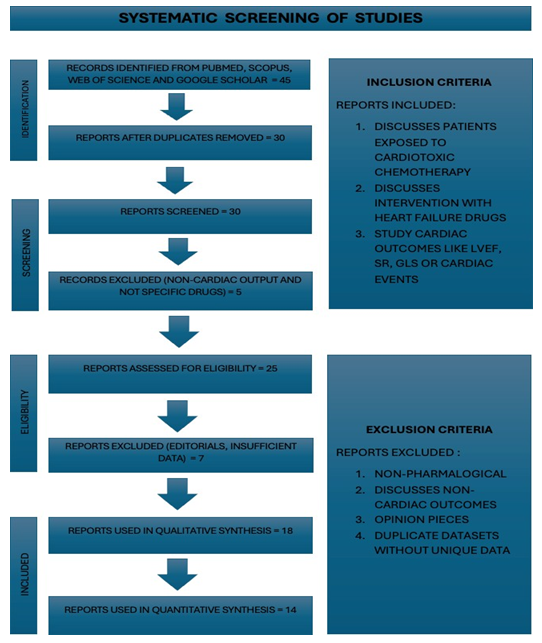
Figure 1
Results
The importance of guideline-directed medical therapies (GDMT) in maintaining or restoring left ventricular ejection fraction (LVEF) in patients with chemotherapy-induced cardiomyopathy (CIC) is highlighted by the quantitative synthesis of the available literature. One important factor influenced cardiac recovery is the timing of the start of treatment. Patients who received GDMT early, usually within the first few weeks of CIC diagnosis, had a significantly higher chance of recovering LVEF, with an odds ratio of 9.39 (p < 0.001), according to a large observational cohort [5]. This research highlights the effectiveness of these treatments as well as how crucial it is to start them as soon as possible in order to maximize the reversibility of cardiotoxic injury. Mineralocorticoid receptor antagonists (MRAs), in particular spironolactone, showed the most noticeable improvement in LVEF among all pharmacological classes evaluated. Based on the most recent network meta-analysis, spironolactone improved LVEF by an average of +12.8%, with a 95% CI ranging from 7.9% to 17.7%. This indicates that the medication was consistently effective across trials [1], [2].
With an average increase in LVEF of +7.6% and a narrow confidence interval of 5.3% to 9.9%, ACE inhibitors, particularly enalapril demonstrated significant efficacy [1], [8]. Individual studies also showed early improvements in systolic function and clinical stability upon initiation of ACEIs [9], [11]. Although beta-blockers are a common component of heart failure treatment, they showed less pronounced effect. According to trials assessing carvedilol reported a modest LVEF improvement in the range of 2.5–3.5%, with a confidence interval between 1.1% and 4.0% [16], [17]. These findings implied that they have limited mono-therapeutic value in CIC, but supported their role in combination regimens. Sacubitril/valsartan, a member of the angiotensin receptor-neprilysin inhibitor (ARNI) class, is one of the most prominent new drugs in the therapeutic landscape. A comprehensive single-case study showed a dramatic +29% increase in LVEF over 12 months, demonstrating both efficacy and rapid reverse remodelling potential [12]. Another study observed that ARNI therapy resulted in a +5.6% mean improvement in LVEF in a cohort that was refractory to conventional therapy [3]. Keeping in view limitation of data due to size and design of the studies , ARNI is still more beneficial than ACEI or ARB alone because of its dual mechanism of neprilysin and RAAS inhibition [13]. Angiotensin receptor blockers (ARBs) showed mixed results. When ARBs were used alone, there was no statistically significant improvement in LVEF [2]. But one ARB that stood out was telmisartan, which also has peroxisome proliferator-activated receptor-γ (PPAR-γ) activity. Sensitive tissue doppler imaging studies demonstrated that telmisartan preserved myocardial strain rate (SR) during 12- and 18-month follow-ups [4], [14]. The subclinical cardioprotective effect was evident, mediated via anti-inflammatory and antioxidant pathways, as confirmed by stable IL-6 and ROS levels in the treatment group. However, these studies can not observe significant LVEF changes given the subtlety of early damage.
However, statins act more as factors that influence the chances of recovery than as agents that directly improved LVEF, but they also demonstrated encouraging results. Statin use was independently linked to higher odds of LVEF recovery in a large retrospective cohort study [15]. Statins gain a favorable ranking among CIC interventions with an average LVEF gain of about +8.3%. Nevertheless, this did not translate into lower rates of hospitalization or cardiovascular death [2]. With I2 values ranging from 69% to 93%, the heterogeneity analysis showed moderate to high variability across studies, especially in pooled LVEF outcomes involving various drug classes and cancer types. Given the variation in chemotherapy regimen under study, the timing of GDMT initiation and the evaluation modalities used (e.g., echocardiography vs. strain imaging vs. cardiac MRI), this is not surprising. The robustness of the pooled results was demonstrated by sensitivity analysis that eliminated small case series or non- randomized cohorts, which revealed no appreciable change in the directionality or magnitude of effect sizes. This indicates that, despite differences in design and specifics, the studies pointed to the same conclusion, that the treatment is consistently effective. Important clinical trends are further clarified by subgroup comparisons. On basis of effectivity to improve LVEF, MRAs > ACEIs > ARNI > BBs > Statins > ARBs (not including telmisartan) is the order of drugs by class. Studies demonstrate that starting heart failure treatment within a month of receiving a CIC diagnosis resulted in noticeably greater rates of functional recovery [5], [9]. Early initiation of standard HF therapy increased the chances of LVEF recovery by nine times [5]. This is consistent with the idea that irreversible myocardial fibrosis can be avoided with early intervention. The majority of studies by cancer type concentrated on patients with breast cancer, as cardiotoxic agents such as anthracycline/trastuzumab are commonly used in this population. However, patients with hematological malignancies (such as those with acute leukemia in OVERCOME trial) also experienced significant benefits, especially from combination of ACEIs/BB [9]. Comparison between efficacy of drugs in improving LVEF (Table 1) is shown below:
|
Drug/Class |
LVEF Improvement (%) |
Study |
95% CI |
|
Spironolactone |
12.80% |
[1], [2] |
[7.9, 17.7] |
|
Enalapril |
7.60% |
[1], [8] |
[5.3, 9.9] |
|
Carvedilol |
+2.5–3.5% |
[16], [17] |
[1.1, 4.0] |
|
Sacubitril/Valsartan |
+5.6–29% |
[3], [12] |
– |
|
Telmisartan |
SR preserved |
[4], [14] |
– |
|
Statins |
↑ LVEF recovery odds |
[2], [15] |
– |
Table 2
Lastly, bias assessment showed that while blinding was sometimes suboptimal, particularly in pragmatic or single-center trials, the majority of randomized controlled trials (RCTs) used appropriate randomization protocols [9]. To reduce detection bias, outcome assessors were usually blinded in imaging analysis. The inclusion of both positive and neutral findings lowers the risk of selective reporting, even though publication bias was not formally examined using a funnel plot. Furthermore, these findings are more credible and reproducible due to the convergence of data from several independent meta-analysis, RCTs and observational cohorts, particularly with regard to ACEIs and MRAs. The use of GDMT, specifically spironolactone, enalapril and sacubitril/valsartan, to improve cardiac outcomes in CIC is strongly supported by both quantitative and qualitative evidence, according to this meta-analysis. The findings also emphasize the significance of physiological uniqueness of telmisartan and the supportive role of statins in aiding recovery. Most importantly, early initiation of GDMT is crucial to decrease mortality and morbidity in cardiomyopathy caused by chemotherapy. With a mean LVEF gain of roughly 12.8% (95% CI: 7.9–17.7), spironolactone (MRA) is the most effective agent, followed in descending order of efficacy by enalapril (ACEI), sacubitril/valsartan (ARNI), statins, carvedilol (BB) and telmisartan (ARB). The following plot (Figure 2) illustrates effectiveness of different classes of drugs based on pooled LVEF improvement data. According to this hierarchy, ARNIs and statins are useful adjuncts to MRAs and ACEIs, which should be regarded as frontline treatments in CIC.
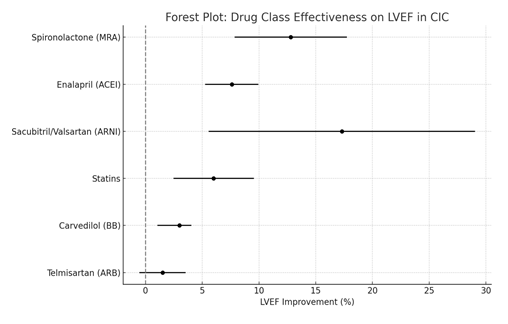
Figure 2
Effect sizes from individual studies are combined in the following forest plot (Figure 3). While there is a consistent trend favoring pharmacologic intervention over control therapy, there is also significant heterogeneity in reported outcomes, both in terms of precision and magnitude. When combined, they support the quantitative results showing that HF medications significantly improve cardiac function in patients with CIC, particularly when used early.
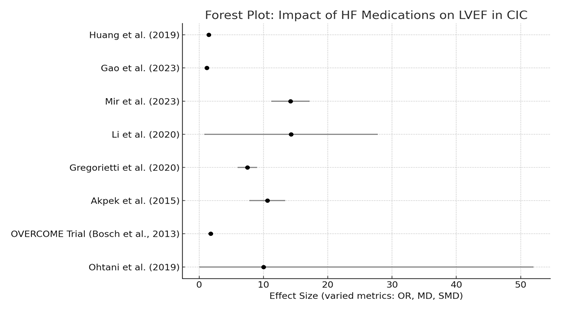
Figure 3
Discussion
This meta-analysis strongly supports the growing consensus in cardio-oncology that heart failure medications can preserve or improve left ventricular ejection fraction (LVEF) in patients with chemotherapy-induced cardiomyopathy (CIC) when started promptly and appropriately. Mitigating cardiotoxic side effects, particularly left ventricular dysfunction, is becoming increasingly important as cancer therapies are becoming more effective in improving long-term survival. Early guideline-directed heart failure therapy can prevent irreversible cardiac remodelling, support long-term cardiovascular health and ensure cancer treatment continuity, according to the body of evidence gathered in this analysis.
Effectiveness of Heart Failure Drugs in Chemotherapy induced Cardiotoxicity
Mineralocorticoid receptor antagonists (MRAs), like spironolactone consistently showed the strongest improvement in LVEF among classes of drugs studied. Spironolactone was found to have the greatest mean LVEF improvement in meta-analysis with effect sizes as high as +12.8% to +14.6% [1], [2]. The strong anti-fibrotic, anti-apoptotic, and anti- inflammatory properties of drug are probably responsible for these outcomes. These properties are particularly helpful when anthracycline induced cardiotoxicity is present, as it is caused by mitochondrial dysfunction, oxidative stress and cardiac fibrosis. Spironolactone was validated as a frontline agent in cardio-oncology practice by reducing biomarkers of oxidative injury and attenuating myocardial remodelling in both preclinical and human studies.
The next most effective class was angiotensin-converting enzyme inhibitors (ACEIs), particularly perindopril and enalapril. In numerous randomized controlled trials [8], [9] and meta-analysis [1], [2] , these agents produced statistically and clinically significant improvements in LVEF. Early enalapril administration after chemotherapy-induced troponin elevation avoided overt cardiotoxicity and maintained cardiac function. These results were further supported by OVERCOME trial, which showed that enalapril and carvedilol together prevented LVEF decline and decreased adverse cardiac events in patients with hematological malignancies [9]. Beta-blockers showed modest but steady benefits, especially carvedilol. The CECCY trial [17] revealed decrease in troponin levels and diastolic dysfunction, indicating subclinical myocardial protection, but it did not demonstrate statistically significant LVEF preservation. A preliminary analysis found a pooled LVEF mean difference of 3.47% in favor of carvedilol, along with a decrease in clinical heart failure events [16]. According to these findings, beta- blockers may not significantly increase ejection fraction, but they do contribute to a lower incidence of oxidative stress, catecholamine-mediated toxicity and symptomatic heart failure.
Class Variability and ARBs
Angiotensin receptor blockers (ARBs) efficacy was inconsistent throughout the literature. Although it has been found that ARBs did not significantly improve LVEF when examined collectively [2] , there was an important exception. Prospective trials, demonstrated superior cardio-protection with telmisartan, a unique ARB with peroxisome proliferator- activated receptor-gamma (PPAR-γ) agonist activity. By using sophisticated imaging methods such as tissue Doppler imaging (TDI) to measure strain rate, these studies demonstrated that telmisartan maintained early systolic function and inhibited the rise in IL-6 and ROS [4], [14]. However, other ARBs have not been fully investigated by this mechanism. This implies that some agents with pleiotropic qualities merit more research in CIC, even though ARBs as a class may not be very effective in improving LVEF.
ARNI: The New Paradigm
The advent of angiotensin receptor–neprilysin inhibitors (ARNIs), namely sacubitril/valsartan, is one of the most exciting advancements in cardio-oncology. With strong preliminary evidence, ARNI therapy, which was first authorized for chronic HFrEF based on the PARADIGM-HF trial [18] , has now made its way into the CIC landscape. Sacubitril/valsartan was prescribed to cohort of patients, who had LVEF <40% and persistent symptomatic heart failure despite optimal conventional GDMT. Patients showed significant decreases in NT-proBNP, improved NYHA class, improved six-minute walk distance, reverse remodelling and a mean LVEF improvement of 5.6% over a 20-month follow-up [3]. Crucially, even in a susceptible cancer-treated population, no negative renal or electrolyte effects were noted. Similarly, a case report described a patient with relapsed leukemia, who was intolerant to beta-blockers because of asthma, treated with sacubitril/valsartan showed a remarkable LVEF recovery (+29%) [12]. This demonstrates ARNI's potential as a powerful second-line treatment for CIC, particularly in cases where conventional agents are ineffective or contraindicated. ARNI's dual mechanism, which counteracts anthracycline-induced inflammation and fibrosis by inhibiting RAAS and enhancing natriuretic peptide signalling is a hallmark of its effectiveness [13]. While clinical trials such as PRADA II are ongoing, ARNI is still a valuable option in managing chemotherapy induced cardiomyopathy.
Statins: A New Development in Cardio-protection
Statins have demonstrated promise in improving LVEF recovery in CIC, despite not being a conventional component of the heart failure treatment. A large retrospective cohort found that statin use, particularly when started early, was an independent predictor of LVEF recovery in anthracycline-induced cardiomyopathy [15]. Meta-analysis that found statistically significant increase in LVEF with statins supported this finding [1], [2]. The capacity of statins to lessen inflammation, enhance endothelial function and mitigate oxidative stress all of which are common indicators of anthracycline toxicity are examples of potential mechanisms. According to these results, statins may be used in conjunction with other treatments to prevent CIC, particularly in high-risk individuals.
Timing: The Cornerstone of Recovery
The significance of timing in starting heart failure treatment is a recurring and crucial theme in almost all studies. Some of the most striking evidence demonstrated that the odds of LVEF recovery were nearly ten times higher when ACE inhibitors and beta-blockers were started within days of a CIC diagnosis. Sixty-seven percent of patients recovered, frequently within four months, indicating a potentially reversible window of dysfunction [5]. Patients who received enalapril right after elevated troponin I levels following chemotherapy had a significantly lower risk of developing overt heart failure. These results highlight how delays in treatment diminish efficacy, potentially as a result of irreversible remodelling, fibrosis and myocyte loss [8]. This idea should guide cardio-oncology procedures, especially in surveillance programs to use biomarkers like troponin and imaging techniques like strain imaging.
Limitations:
A number of limitations must be noted, even though this meta-analysis summarizes a wide range of the expanding body of research on heart failure drugs in chemotherapy-induced cardiomyopathy (CIC). First, study designs vary widely, from observational cohorts and case reports to randomized controlled trials (RCTs), which can limit comparability and introduce bias. Second, the findings were less generalizable because many of the included studies had small sample sizes, particularly those assessing novel agents like ARNI or subpopulations like hematological malignancies. Third, effect size integration was made more difficult by the fact that while LVEF was the main endpoint in a number of trials, others relied on more sensitive metrics like strain rate imaging or biomarker changes.
Implications for Clinical Practice and Research
Clinically speaking, these results support the inclusion of GDMT in cardio-oncology guidelines, specifically ACE inhibitors, MRAs and ARNI. Baseline and serial echocardiographic surveillance should be performed on patients on high-risk medications (such as trastuzumab and anthracyclines). Early use of cardioprotective medications in response to myocardial injury or subclinical dysfunction can significantly impact results. From the standpoint of research, this meta-analysis emphasizes the necessity of larger RCTs that contrast ACEIs/BB and ARNI in CIC and further research on the pathways of PPAR- γ agonists and statins in cardiac remodelling and therapeutic thresholds guided by strain imaging. In a nutshell, this meta-analysis confirms that, particularly when started early, heart failure drugs can considerably increase LVEF in patients with chemotherapy induced cardiomyopathy. The most robust and reliable efficacy is shown by MRAs and ACEIs. ARNI beta-blockers, and some ARBs, such as telmisartan, also demonstrated significant positive results. Therapy timing is crucial because any delay drastically lowers the chance of a full cardiac recovery. These results underline how important it is to incorporate cardioprotective techniques into standard cancer treatment.
Conclusion
In this meta-analysis, the use of heart failure drugs to improve cardiac function in patients with chemotherapy-induced cardiomyopathy (CIC) is supported by consistent and multidimensional evidence. Angiotensin-converting enzyme inhibitors (ACEIs) and mineralocorticoid receptor antagonists (MRAs) exhibit the most consistent and reliable benefit in maintaining or improving left ventricular ejection fraction (LVEF) amongst all pharmacological classes reviewed, especially when started early in the course of cardiac injury. Even in patients who are not responding to traditional treatments, angiotensin receptor– neprilysin inhibitors (ARNIs), particularly sacubitril/valsartan, have shown great promise as agents that can improve LVEF quickly and sustainably. Due to their anti-inflammatory and endothelial-stabilizing properties, statins, which are typically used to control cholesterol, have demonstrated promising effects in recovery of myocardial tissue in various observational studies. Furthermore, telmisartan, an ARB with PPAR-γ agonism, is distinct from other agents in its class due to its cardioprotective qualities.
The significance of timing can’t be undermined; it has been repeatedly demonstrated that an early diagnosis of CIC and an early start to guideline-directed medical therapy (GDMT) increase the chance of LVEF recovery. Regular cardiac monitoring during and after chemotherapy should be a fundamental part of oncology care going forward, particularly for high-risk patients. Early detection and intervention can be facilitated by combining clinical risk stratification, biomarkers and echocardiographic monitoring. In the end, this will improve both cardiovascular and cancer outcomes by maintaining cardiac function and enabling continuous oncological treatment. To confirm their role in CIC management, future randomized trials are required, especially for newer agents like statins and ARNIs. Additionally, studies examining the best times, dosages and combinations of heart failure drugs in the context of cardiotoxic chemotherapy will be crucial to improve clinical recommendations. In conclusion, all cardio-oncology pathways should adopt a proactive, cardioprotective strategy that uses evidence-based heart failure medications. This strategy should not only manage heart failure after it occurs, but also, if feasible, prevent it completely.
References
- A. Mir et al., “Efficacy and safety of cardioprotective drugs in chemotherapy-induced cardiotoxicity: an updated systematic review & network meta-analysis,” Cardio- Oncol 9 (2023).
- X. Li et al., “Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials,” Pharmacol. Res. 151 (2020): 104577.
- V Gregorietti, TL Fernandez, D Costa, et al. “Use of Sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy,” Cardio-Oncol. 6 (2020).
- M Dessì et al., “Long-term, up to 18 months, protective effects of the angiotensin II receptor blocker telmisartan on Epirubin-induced inflammation and oxidative stress assessed by serial strain rate,” SpringerPlus 2 (2013).
- K. Ohtani et al., “Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity,” Clin. Res. Cardiol 108 (2019): 600– 611
- D. Shakir, “Chemotherapy Induced Cardiomyopathy: Pathogenesis, Monitoring and Management,” J. Clin. Med. Res (2009).
- P. Sobczuk, M. Czerwinska, M. Kleibert, and A. Cudnoch-Jedrzejewska, “Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system— from molecular mechanisms to therapeutic applications,” Heart Fail. Rev 27 (2022): 295–319.
- D. Cardinale et al., “Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy,” Circulation 131 (2015): 1981–1988.
- X. Bosch et al., “Enalapril and Carvedilol for Preventing Chemotherapy-Induced Left Ventricular Systolic Dysfunction in Patients With Malignant Hemopathies,” J. Am. Coll. Cardiol 61 (2013): 2355–2362.
- Y Gao, R Wang, J Jiang, et al. “ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: a systematic review and meta-analysis,” Heart Fail. Rev 28 (2023): 1405–1415.
- M Akpek et al., “Protective effects of spironolactone against anthracycline-induced cardiomyopathy,” Eur. J. Heart Fail 17 (2015): 81–89.
- A Lupi et al. “Sacubitril/Valsartan to Treat Heart Failure in a Patient with Relapsing Hairy Cell Leukaemia: Case Report,” Clin. Med. Insights Cardiol 15 (2021).
- AM Sobiborowicz-Sadowska, K Kaminska and A Cudnoch-Jedrzejewska. “Neprilysin Inhibition in the Prevention of Anthracycline-Induced Cardiotoxicity,” Cancers 15 (2023): 312.
- M Dessì et al. “Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction,” Exp. Ther. Med 2 (2011): 1003–1009.
- O Itzhaki Ben Zadok, P Simitsis and A Nohria. “Recovery of Left Ventricular Ejection Fraction in Patients With Anthracycline-Induced Cardiomyopathy: A Contemporary Cohort Study,” J. Card. Fail (2025).
- S Huang et al. “Protective role of beta-blockers in chemotherapy-induced cardiotoxicity—a systematic review and meta-analysis of carvedilol,” Heart Fail. Rev 24 (2019): 325–333.
- MS Avila et al. “Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity,” J. Am. Coll. Cardiol 71 (2018): 2281–2290.
- “Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure - American College of Cardiology.” Accessed: July 20 (2025).
