Risk Factors for Mortality in Older Adults with Pleural Effusion
Gal Oren, Sari Tal*
Acute Geriatrics Department at Kaplan Medical Center, Rehovot. Affiliated with the Hebrew University of Jerusalem, Israel
*Corresponding authors: Sari Tal, Acute Geriatrics Department at Kaplan Medical Center, Rehovot. Affiliated with the Hebrew University of Jerusalem, Israel.
Received: 16 April 2025; Accepted: 28 April 2025; Published: 19 July 2025
Article Information
Citation: Gal Oren, Sari Tal. Risk Factors for Mortality in Older Adults with Pleural Effusion. Journal of Biotechnology and Biomedicine. 8 (2025): 271-277.
DOI: 10.26502/jbb.2642-91280193
Share at FacebookAbstract
Background and objective: Pleural effusion (PE) is often classified as either a transudative or an exudative process. We aimed to examine the causes of pleural effusions and the short- and long-term survival in the older patients hospitalized in the acute geriatric and internal medicine wards. Methods: Patients aged ≥ 65 years, who underwent thoracentesis, were included in this retrospective study. To distinguish between exudative and transudative PE, the Light’s criteria were applied. We compared medical indices and mortality in patients with exudative PE to those with transudative effusion according two age groups (65 - 85 vs. > 85 years). Results: The rates of the main etiologies of PE were congestive heart failure (CHF), 46%, lung infections, 25%, and malignancy, 12%, of the whole study population. Lung infections accounted for 53% of the exudative PE patients, with a higher presence among the >85 group of patients. The etiology of transudative PE was primarily CHF, more common among the patients of the >85 group. In malignancy, CHF and lung infections patients, the chances of dying within one year were 80.3%, 63.2%, and 45.5%, respectively. In transudative PE patients, ten-year survival of the 65-85 group of patients was significantly higher. In the exudative PE patients, there was no difference in survival between the two age groups. Right-sided and bilateral pleural effusions were associated with worse prognosis. Conclusion: Clinicians encountering patients with PE should be aware of their heightened mortality risks, and should carefully monitor their underlying etiology and employ appropriate optimal management.
Keywords
Pleural effusion; Older adults; Congestive heart failure; Lung infections; Malignancy; Mortality
Pleural effusion articles; Older adults articles; Congestive heart failure articles; Lung infections articles; Malignancy articles; Mortality articles
Article Details
Introduction
Pleural effusions (PEs) are a common clinical problem in medical practice. PEs result from the accumulation of fluid in the pleural space surrounding the lungs [1]. A pleural effusion (PE) describes an excess of fluid in the pleural cavity, usually resulting from an imbalance in the normal rate of pleural fluid production or absorption, or both [2]. They develop when more fluid enters the pleural space than is removed. Potential mechanisms of pleural fluid accumulation include, among others, increased interstitial fluid in the lungs secondary to increased pulmonary capillary pressure (i.e., heart failure) or permeability (i.e., pneumonia); decreased intrapleural pressure (i.e., atelectasis); decreased plasma oncotic pressure (i.e., hypoalbuminemia); and increased pleural membrane permeability and obstructed lymphatic flow (e.g., pleural malignancy or infection) [3]. The cause of PE is often classified initially as either a transudative or an exudative process, with the former usually associated with cardiac (heart failure), renal, or hepatic dysfunction (cirrhosis), and the latter with conditions that cause an excess of inflammation, such as malignancy or infection [2-4]. Transudative effusion results from imbalances in hydrostatic and oncotic forces. It occurs when fluid permeates the pleural cavity via intact pulmonary vessels. In contrast, exudative effusion refers to fluid escaping into the pleural space through lesions in blood and lymph vessels due to inflammation. Exudative effusions occur when the local factors influencing the accumulation of pleural fluid are altered. Pneumonia, malignancy, and thromboembolism account for most exudative effusions in the United States [3]. Malignant PEs are known to be associated with high mortality, but there is increasing evidence that patients with non-malignant PEs also carry a high mortality rate, especially when the PEs are related to congestive heart failure (CHF) [5]. In clinical practice, exudative effusions can be separated effectively from transudative effusions using Light’s criteria. These criteria classify an effusion as exudate if one or more of the following are present: (1) the ratio of pleural fluid protein to serum protein is greater than 0.5; (2) the ratio of pleural fluid lactate dehydrogenase (LDH) to serum LDH is greater than 0.6; or (3) the pleural fluid LDH level is greater than two thirds of the upper limit of normal for serum LDH [3]. Many causes of PEs are recognized, crossing a wide variety of diseases [2]. Epidemiological data on the causes of PEs are scarce [6]. There is also little in the literature regarding mortality of patients with nonmalignant Pes [7]. We have studied the causes of PEs in two age groups of older patients hospitalized in the acute geriatric and internal medicine wards, and calculated ten-year survival of transudative vs. exudative PE.
Material and Methods
Study design, setting and population
We retrospectively surveyed electronic hospital health records of 700 older patients, aged ≥ 65 years old, admitted between the year 2008 and 2018 to the acute geriatric and internal medicine wards, and underwent thoracentesis. The thoracentesis was performed by using ultrasound guidance (Vscan with dual Probe, Software version 1.4, General Electric, Norway). To distinguish between exudative and transudative pleural effusion, the Light’s criteria were applied [8]. Sixty-five patients, in whom some criteria were missing or the results were unclear were excluded. The remaining 635 patients were assigned either to exudative or transudative pleural effusion group. In each group, the patients were divided into two age groups, 65 - 85 and > 85 years old. The retrieved data included patient demographics, medical diagnoses, laboratory results and clinical outcomes. We compared mortality in the two age groups of patients (65 - 85 vs. > 85years), with exudative PE to those with transudative PE.
The study was approved by the Institutional Ethics Committee of the Kaplan Medical Center, Rehovot, Israel.
Statistical analysis
Data were analyzed, using SPSS version 29.0 program. Categorical and nominal variables were analyzed, using Pearson's chi-square (χ²) test. A comparison was performed between two age groups using both the chi-square test and the Fisher's exact test. Continuous variables were presented as means ± standard deviations (SD). The normality of these variables was assessed, using the Kolmogorov-Smirnov test. In cases, in which abnormal distribution was observed, non-parametric tests were conducted. The Mann-Whitney test was used to compare the two age groups. Kaplan-Meier curves were used to describe survival of transudative and exudative pleural effusion older adults within ten years post thoracentesis. The probability of mortality within one year for the disease categories - CHF, pulmonary infection, and malignancy - was calculated, using the following methodology: for each category, only patients for whom the number of days from thoracentesis to the date of death was less than or equal to 365 were selected. The number of patients meeting this criterion was divided by the total number of patients in that category, and the result represents the probability of mortality. By Kaplan Meier analysis, in older adults suffering from malignancy, CHF and lung infections one-year survival post thoracentesis was calculated. In addition, quantitative assessment of the risk of death relative to malignancy was performed by Cox regression analysis. P value < 0.05 was considered statistically significant.
Results
Patient characteristics
Selected characteristics of the 653 patients (46% females), stratified according to PE diagnosis and two age groups (65 - 85 and > 85 years), are presented in Table 1. Of the whole group of patients, 47.9% suffered from exudative PE. The mean age of the exudative PE group was 77.0 ± 5.3 in the 65 - 85 and 89.0 ± 7.00 in the > 85 group. The mean age of the transudative PE group was 77.9 ± 4.9 in the 65 - 85 and 89.6 ± 3.70 in the > 85 group. Smoking was more common in the younger group of patients in both the exudative and the transudative PE patients.
|
Characteristic |
Exudate |
|
p-value |
Transudate |
|
p-value |
|
n=304 |
n=331 |
|||||
|
65-85 |
>85 |
65-85 |
.>85 |
|||
|
n=216 |
n=88 |
n=184 |
n=147 |
|||
|
Age, mean ± SD |
76.13±5.52 |
88.89±10.38 |
<0.001 |
77.94±4.86 |
89.59±3.72 |
<0.001 |
|
Female, n% |
96(44.4) |
44(50.0) |
0.378 |
94(51.1) |
68(46.3) |
0.383 |
|
Smoking, n(%) |
45(20.8) |
6(6.8) |
0.003 |
25(13.6) |
9(6.1) |
0.026 |
|
Length of hospital stay, mean ± SD |
10.47±12.79 |
10.48±8.91 |
0.25 |
18.56±73.11 |
11.07±10.38 |
0.811 |
|
In-hospital mortality |
32(14.8) |
11(12.5) |
0.377 |
40(21.7) |
38(25.9) |
0.381 |
|
Comorbidities, n(%) |
||||||
|
Ischemic heart Disease |
67(31.0) |
25(28.4) |
0.653 |
64(34.8) |
58(39.5) |
0.381 |
|
CHF |
33(15.3) |
26(29.5) |
0.004 |
71(38.6) |
62(42.2) |
0.508 |
|
COPD |
44(20.4) |
17(19.3) |
0.835 |
39(21.2) |
35(23.8) |
0.571 |
|
Dyslipidemia |
90(41.7) |
25(28.4) |
0.031 |
80(43.5) |
33(22.4) |
<0.001 |
|
Diabetes Mellitus |
99(45.8) |
21(23.9) |
<0.001 |
93(50.5) |
46(31.3) |
<0.001 |
|
Hypertension |
146(67.6) |
73(83.0) |
0.007 |
139(75.5) |
117(79.6) |
0.372 |
|
CVA |
40(18.5) |
12(13.6) |
0.305 |
37(20.1) |
38(25.9) |
0.215 |
|
CRF |
57(26.4) |
22(25.0) |
0.802 |
60(32.6) |
45(30.6) |
0.698) |
|
Atrial fibrillation |
34(15.7) |
26(29.5) |
0.006 |
66(35.9) |
64(43.5) |
0.156 |
|
Dementia |
17(7.9) |
17(19.3) |
0.004 |
18(9.8) |
32(21.8) |
0.002 |
|
Parkinson |
13(6.0) |
4(4.5) |
0.612 |
9(4.9) |
12(8.2) |
0.225 |
|
History of carcinoma |
89(41.2) |
27(30.7) |
0.087 |
44(23.9) |
41(27.9) |
0.41 |
|
Effusion side |
||||||
|
Right |
77(35.6) |
34(38.6) |
0.841 |
62(33.7) |
45(30.6) |
0.794 |
|
Left |
68(31.5) |
25(28.4) |
0.841 |
23(12.5) |
21(14.3) |
0.794 |
|
Bilateral |
71(32.9) |
29(33.0) |
0.841 |
99(53.8) |
81(55.1) |
0.794 |
|
Laboratory tests |
||||||
|
Urea |
65.22±52.61 |
70.96±52.48 |
0.078 |
81.91±54.34 |
84.96±50.25 |
0.211 |
|
Creatinine |
1.36±1.32 |
1.25±0.96 |
0.538 |
1.53±1.22 |
1.45±0.95 |
0.402 |
|
Albumin |
3.11±0.64 |
3.04±0.54 |
0.276 |
3.05±0.60 |
3.05±0.57 |
0.885 |
|
Cholesterol |
148.15±44.44 |
141.94±41.73 |
0.288 |
132.39±33.69 |
137.42±35.51 |
0.204 |
|
LDH |
568.85±368.10 |
514.11±242.56 |
0.467 |
606.49±318.48 |
580.78±295.06 |
0.416 |
|
CHF – Congestive Heart Failure; COPD – Chronic Obstructive Pulmonary Disease; CVA – cerebrovascular accident; CRF – chronic renal failure; LDH - lactate dehydrogenase. |
||||||
Table 1: Univariate analysis of study selected patient characteristics by pleural effusion (N = 635).
Comorbidities
In the study PE patients, the most common comorbidities were CHF, dyslipidemia, diabetes mellitus, hypertension, atrial fibrillation, and dementia. In the exudative PE group, except for dyslipidemia and diabetes mellitus, the percentage of the older group with the mentioned comorbidities was higher (Table 1). In the transudative PE group, as in the exudative PE group, dyslipidemia and diabetes mellitus were more common in the younger group. There was no difference in the presence of the other comorbidities between the two age groups, except for dementia, which was more common in the older group. The exudative and the transudative PE patients did not differ in the laboratory tests results as well as the length of in-hospital stay and the in-hospital mortality rates.
PE etiologies in exudative patients
Of the whole study population, the rates of the primary PE etiologies were CHF, lung infections and malignancy, 46%, 25% and 12%, respectively (Table 1). Lung infections (including pneumonia, empyema and respiratory infections) accounted for 53% of the exudative PE patients, with a higher presence (63.4% vs. 48.1%) among the > 85 age group of patients. Malignancy played a smaller role (24.3% of the exudative PE patients), with no difference between the two age groups.
PE etiologies in transudative patients (Table1)
The etiology of transudative PE was primarily CHF (87.9% of the transudative PE patients), more common (91.8% vs. 84.8%) among the patients of the > 85 age group. Liver failure and renal failure much less contributed to transudative PE (4.8% of the transudative PE patients), more common (13.3% vs. 7.4%) among the 65 - 85 age group of patients (data not shown).
Unilateral and bilateral PE effusions
Patients with R-sided PE, were older (82 y vs. 80 y) than those with L-sided effusion, their in-hospital length of stay was longer (14.5 vs. 10 days), and the number of days to death was lower. In addition, their blood urea levels were higher (80 vs. 66 mg/dL) and albumin levels were lower (3.06 vs. 3.09 g/dL), Table 2. Bilateral PE was present in 32.5%, 19.3% and 5.7% of CHF, lung infections and malignancy patients, respectively (data not shown). In comparison between unilateral vs. bilateral PE, in the latter albumin levels were lower (2.54 vs. 3.07 g/dL) and the length of stay was longer (24.6 vs. 12.7 days). The number of days to death in the bilateral PE patients was almost significantly lower than in those with the unilateral one (179.3 vs. 455.8 days), Table 3. Kaplan Meier analysis did not find difference in one-year survival between bilateral and unilateral PE (data not shown).
|
|
L-side |
R-side |
p-value |
|
Parameters |
Mean (STD) |
|
|
|
Age |
80.59±9.13 |
82.16±8.71 |
0.019 |
|
Urea |
66.32±45.64 |
79.72±54.76 |
0.017 |
|
Creatinine |
1.24±0.95 |
1.5±1.25 |
0.141 |
|
Albumin |
3.09±0.56 |
3.06±0.61 |
0.001 |
|
Cholesterol |
140.80±38.94 |
139.78±40.28 |
0.684 |
|
LDH |
548.38±288.25 |
599.23±348.84 |
0.682 |
|
Length of hospital stay |
9.83±10.36 |
14.52±49.58 |
0.001 |
|
Days to death |
520.24±785.88 |
415.89±695.62 |
0.038 |
|
LDH - lactate dehydrogenase. |
|||
Table 2: Comparative Analysis of Demographic and Clinical Parameters in Patients with L-Sided vs. R-Sided pleural effusion.
|
|
L/R-side |
Bilateral |
p-value |
|
Parameters |
Mean ± SD |
|
|
|
Age |
81.54±8.90 |
79.32±8.48 |
0.276 |
|
Urea |
74.42±51.73 |
94.45±67.10 |
0.094 |
|
Creatinine |
1.40±115 |
1.53±1.07 |
0.359 |
|
Albumin |
3.07±0.59 |
2.54±0.75 |
0.002 |
|
Cholesterol |
140.19±39.72 |
135.11±45.60 |
0.71 |
|
LDH |
579.06±326.88 |
570.55±311.98 |
0.872 |
|
Length of hospital stay |
12.66±39.14 |
24.60±24.77 |
0.005 |
|
Days to death |
455.87±732.67 |
179.37±408.64 |
0.058 |
|
LDH - lactate dehydrogenase. |
|||
Table 3: Comparative Analysis of Demographic and Clinical Parameters in Patients with L/R-Sided vs. bilateral pleural effusion.
Survival and mortality
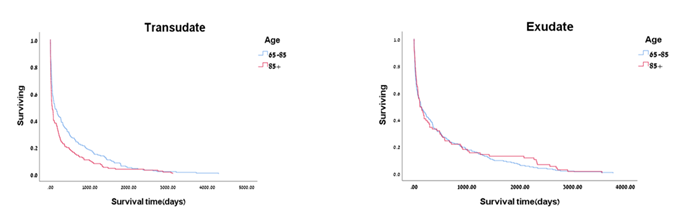
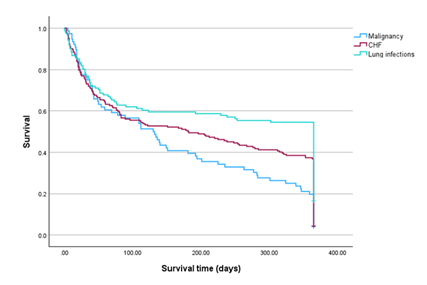
In malignancy, CHF and lung infections patients, the percentage of patients who died within one year post thoracentesis was 80.3%, 63.2%, and 45.5%, respectively (Table 4). Cox regression analysis determined the impact of each disease. Malignancy mostly affected mortality. Mortality from the other two diseases was equally significantly lower by 25% (HR=0.751, 95%CI 0.647-0.872), (Table 5). By Kaplan Meier analysis, the impact on mortality in exudative and transudative PE, within the two age groups, is illustrated in Fig.1. In transudative PE patients, ten-year survival of the 65 - 85 age group of patients was significantly higher. In the exudative PE patients, there was no difference in survival between the two age groups. In the transudative PE 65 - 85 and > 85 age group of patients, the probability of survival within one year post thoracentesis was 34.6% and 21%, respectively. In the exudative PE 65 - 85 and > 85 age group of patients, there was no difference in the probability of survival within one year post thoracentesis (33.9% and 32.9%, respectively). By Kaplan Meier analysis, in older adults suffering from malignancy, one-year survival was the lowest, followed by CHF and lung infections (Fig. 2). The probability of survival within one year post thoracentesis was 19.74%, 36.81%, and 54.55% in malignancy, CHF, and lung infections patients, respectively.
|
Diseases |
Died |
Survived |
p-value |
|
n(%) |
|||
|
Malignancy, n = 76 |
61(80.3) |
15(19.7) |
|
|
Lung infections, n = 161 |
71(43.5) |
90(56.5) |
<0.001 |
|
CHF, n = 192 |
122(63.5) |
70(36.5) |
Table 4: Mortality within one year in malignancy, lung infections and CHF patients.
|
Disease |
Mortality (%) |
Hazard Ratio (HR) |
95%CI |
|
Malignancy |
80.3 |
Reference |
|
|
1 |
|||
|
Lung infections |
43.5 |
0.751 |
0.647-0.872 |
|
CHF |
63.5 |
0.751 |
0.647-0.872 |
Table 5: Mortality within one year in malignancy, lung infections and CHF patients by Cox regression.
Discussion
There are limited epidemiological data on the causes of PE [6]. Although many different diseases may cause PE, the most common causes in adults are heart failure, malignancy, pneumonia, tuberculosis, and pulmonary embolism [3]. In our study, similarly to some other studies [6,7], the most prevalent cause of PE was CHF (46%), followed by lung infections (25%), and malignancy (12%). In a non-published Spanish study in octogenarians, heart failure accounted for more than half of all Pes [9]. In CHF patients, in our study, mortality rate within one year post thoracentesis was 63.2%. In an American study, CHF complicated by PE had 53% one-year mortality [7]. In a British study in younger patients (68 years old) with CHF, the one-year mortality rate was 50% [10]. Exudative PE is most likely to be associated with pneumonia [2]. Pneumonia is the most common etiology of exudative pleural infections, occurring in up to 9% of patients with pneumonia [7,11]. The worldwide incidence of pleural infections has been increasing. An actual increase of the disease may be due to changes in the age distribution of the population, as older age groups are becoming more prominent in many developed countries, and advanced age is associated with several diseases that may predispose to the development of pleural infections. Additionally, male sex, alcoholism, and low socioeconomic status, as well as malignancies and other common comorbidities are associated with higher risk for the development of pleural infections [11]. PE in the setting of pulmonary infection is a poor prognostic sign and is one of the major causes of pulmonary mortality and morbidity.[7,12] In lung infection patients, in our study, mortality rate within one year post thoracentesis was 45.5%, higher than the finding in a study performed in younger older adults, in whom there was a 26% one-year mortality [7]. However, in a Finnish study of a younger group of patients, 60% of deaths occurred in patients suffering from pulmonary infection complicated by PE. Possible reason for higher mortality rate might be a high prevalence of significant comorbidities in the non-survivors [11]. Malignant PE (MPE) is the second most common exudative PE cause [8]. It is a common clinical problem that can be seen in 15% to 35% of patients diagnosed with cancer [13,14]. MPE, in our study, accounted for about 24.3% of exudative PE patients. Generally, MPE is associated with poor outcomes [7]. In our study, in malignancy pleural effusion patients 80.3% died within one year post thoracentesis, similarly to the findings in American and Turkish studies 77% and 84%, respectively [7,13]. In two studies, British and Estonian, in younger MPE patients, one-year mortality rate was 70% and 61%, respectively [10,15]. In our study, Kaplan Meyer analysis showed that the lowest survival was in MPE. Cox regression analysis strengthened this finding, as it showed that, in comparison with CHF and lung infections, malignancy had the strongest association with one year mortality.
To our knowledge, there is no study comparing the one- and ten-year survival of transudative and exudative PE in two age groups of older patients. In our study, in the transudative PE 65 - 85 and > 85 age group of patients, the probability of survival within one year post thoracentesis was 34.6% and 21%, respectively. In the exudative PE 65-85 and > 85 age group of patients, there was no difference in the probability of survival within one year post thoracentesis, 33.9% and 32.9%, respectively. Maybe, because exudative PEs include malignancy diseases, which often have worse prognosis than transudative PEs, younger age might not be protective, as indeed described in a Finnish study of a younger group of older patients [11]. Right-sided PE was associated with older age, longer hospital stay length, lower albumin level, higher urea level, and lower survival compared to left-sided PE. To our knowledge, there is no study comparing the health condition of patients suffering from right-sided PE to those with left-sided one. Maybe right-sided effusion is more common in severely ill patients, but as it is a retrospective study, we could not identify them.
Bilateral effusions, in our study, accounted for 44% of pleural effusion patients. Bilateral pleural effusions occur in 15% of the cases in non-critically ill patients, and up to 55% of those in the intensive care population [1,7]. The etiology of bilateral PE includes, among others, CHF, liver and renal failure, and malignancy [7]. In our study, bilateral effusions were present in 32.5%, 19.3% and 5.7% of CHF, lung infections and malignancy cases, respectively. In two studies, Polish and Spanish, higher rates of patients, suffering from CHF, presented with bilateral PE, 51% and 61%, respectively [6,9].
The association between bilateral PE and mortality has been previously reported in few studies [7]. In our study, bilateral PE was almost significantly associated with higher mortality than the unilateral one. However, by Kaplan Meier analysis no significant difference was found between bilateral and unilateral PE. In the Walker et al., study, in non-malignant bilateral exudative and transudative PEs, the risk to die within one year was higher by 3.55 and by 2.78, respectively. Non-malignant bilateral exudative and transudative PEs were associated with a worse prognosis than the unilateral ones, with a 57% and 43% one-year mortality, respectively [10]. In an Israeli study, in acute pulmonary embolism patients, those with bilateral PE had a higher probability for in-hospital death in comparison with patients with unilateral PE [16]. In an American study, bilateral PE was markedly associated with high mortality and had a significantly higher risk of death. Caregivers encountering patients with diseases complicated by PE should be aware of their heightened mortality risks and aggressive management of the underlying etiology is warranted [7].
Limitations of the study
Our study has all the disadvantages of a retrospective observational study. We did not have all the data that may have been influencing mortality, because they were not found in the patients' electronic records. Our study strength lies in its population relatively large size. Since the study focuses on short- and long-term mortality due to diseases complicated by PE in older patients, including the oldest-old, we would like to point out that we have contributed to the medical knowledge concerning this population, whose proportion in the geriatric population is recently significantly increasing.
Conclusion
Older PE patients undergoing thoracentesis have high short- and long-term mortality. Although mortality rate was highest in MPE patients, mortality in non-malignant PE in older adults was considerable. Therefore, PE should be considered as a poor prognostic sign. Clinicians encountering patients with PE should be aware of their heightened mortality risks, and should carefully monitor their underlying etiology and employ appropriate optimal management.
Ethics approval
The study was approved by the Institutional Ethics Committee of the Kaplan Medical Center, Rehovot, Israel. In this study, patient consent was waived due to its retrospective nature of the study.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
I would like to thank Felicia Stern, PhD. RD for her assistance in the preparation and editing of this manuscript.
References
- Puchalski JT, Argento AC, Murphy TE, et al. Etiologies of bilateral pleural effusions. Resp Med 107 (2013): 284-291.
- Bhatnagar, R, Maskell N. The modern diagnosis and management of pleural effusions. BMJ 351 (2015): h4520.
- Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Phys 73 (2006): 1211-1220.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int 116 (2019): 377–386.
- Wijayaratne T, Yousuf A, Panchal R. Cardiac related pleural effusions: a narrative review. J Thorac Dis 16 (2014): 1674-1686.
- Korczynski P, Górska, K, Konopka D, et al. Significance of congestive heart failure as a cause of pleural effusion: Pilot data from a large multidisciplinary teaching hospital. Cardiol J 27 (2020) : 254-261.
- DeBiasi EM, Pisani MA, Murphy TE, et al. Mortality among patients with pleural effusion undergoing thoracentesis. Eur Resp J 46 (2015): 495-502.
- Light RW. Pleural effusions. Med Clin N Am 95 (2011): 1055-1070.
- Porcel J. Pleural effusions from congestive heart failure. Sem Respir Crit Care Med 31 (2011): 689-697.
- Walker SP, Morley AJ, Stadon L, et al. Nonmalignant pleural effusions: A prospective study of 356 consecutive unselected patients. Chest 151 (2017): 1099-1105.
- Lehtomäki A, Nevalainen R, Ukkonen M, et al. Trends in the incidence, etiology, treatment, and outcomes of pleural infections in adults over a decade in a Finnish University Hospital. Scand. J Surg 109 (2020): 127-132.
- Krishna R, Antoine MH, Rudrappa M. Pleural effusion. In: StatPearls [Internet]. Treasure Island (FL). StatPearls Publishing (2024).
- Ermin S, Özdogan Y, Batum O, et al. The role of LENT and PROMISE scores in predicting survival in malignant pleural effusion. Lung India. 39 (2022): 325-330.
- Herrera Lara S, Fernández-Fabrellas E, Juan Samper G, et al. Predicting malignant and paramalignant pleural effusions by combining clinical, radiological and pleural fluid analytical parameters. Lung 195 (2017): 653-660.
- Laisaar T, Palmiste V, Vooder T, et al. Life expectancy of patients with malignant pleural effusion treated with video-assisted thoracoscopic talc pleurodesis. Interact Cardiovasc Thorac Surg 5 (2006): 307-310.
- Levy O, Fux D, Bartsikhovsky T, et al. Clinical relevance of bilateral pleural effusion in patients with acute pulmonary embolism. Inter Med J 50 (2020): 938-944.