Safety and Blood Pressure Outcomes of Renal Denervation for Resistant Hypertension: Meta-Analysis of Randomized Trials
Arhum Mahmood1, Mohamed Aboelmaaty2, Ghazala S. Virk3, Sumaiya Khan4, Abdul Hanan5, Zain Bin Saeed6, Sara Zubair Ahmed7, Muhammad Ahmer Sohaib8, Ahmad Sheraz Iqbal9, Binish Essani10, Marium Abid11, Muhammad Sohail S. Mirza12*
1MD, Henry Ford Health System, Detroit, USA
2MD, University of Minnesota, Minnesota
3MD, Avalon University School of Medicine, Willemstad, Curacao
4MBBS, Khaja Bandanawaz Institute of Medical Sciences, Karnataka, India
5MBBS, Khawaja Muhammad Safdar Medical College, Sialkot, Pakistan
6MBBS, Allama Iqbal Medical College, Lahore, Pakistan
7MBBS, Baqai Medical and Dental University, Karachi, Pakistan
8MBBS, Allama Iqbal Medical College, Lahore, Pakistan
9MBBS, Allama Iqbal Medical College, Lahore, Pakistan
10MBBS, Jinnah Medical and Dental College, Karachi, Pakistan
11MD, Jinnah Medical and Dental College, Karachi, Pakistan
12MBBS, Shandong University School of Medicine, Jinan, China
*Corresponding author: Muhammad Sohail S. Mirza, MBBS, Shandong University School of Medicine, Jinan, China.
Received: 14 July 2025; Accepted: 22 July 2025; Published: 29 July 2025
Article Information
Citation: Mahmood A, Aboelmaaty M, Virk GS, Khan S, Hanan A, Saeed ZB, Ahmed SZ, Sohaib MA, Iqbal AS, Essani B, Abid M, Mirza MSS. Safety and Blood Pressure Outcomes of Renal Denervation for Resistant Hypertension: Meta-Analysis of Randomized Trials. Cardiology and Cardiovascular Medicine. 9 (2025): 287-295.
Share at FacebookAbstract
Background: Catheter-based renal denervation (RDN) is a potential nonpharmacological treatment for resistant hypertension, though its efficacy and safety require further validation. This meta-analysis compared RDN to sham procedures on blood pressure (BP) outcomes and safety.
Methods: We pooled data from 6 randomized controlled trials (999 patients) using random-effects models. Primary outcomes were changes in 24-hour ambulatory systolic/diastolic BP (SBP/DBP) and office SBP. Safety assessed adverse events and renal function. Heterogeneity was evaluated via I².
Results: RDN significantly reduced 24-hour ambulatory DBP (SMD: -0.23; 95% CI: -0.47, -0.00; p=0.05) and office SBP (SMD: -0.29; 95% CI: -0.50, -0.08; p=0.007) versus sham. The reduction in 24-hour ambulatory SBP was not significant (SMD: -0.21; 95% CI: -0.47, 0.05; p=0.11). Adverse event rates and renal function changes were similar between groups (p=0.86). Analyses showed moderate-to-high heterogeneity (I²=62%-69%).
Conclusions: RDN effectively lowers ambulatory DBP and office SBP with a favorable safety profile. Its effect on ambulatory SBP remains uncertain. While a promising option for resistant hypertension, standardized protocols and long-term data are needed to confirm efficacy and durability.
Keywords
Renal Denervation (RDN); Treatment-Resistant Hypertension; Blood Pressure Reduction; Catheter-Based Intervention; Radiofrequency Ablation; Ultrasound Ablation; Ambulatory Blood Pressure Monitoring (ABPM); Cardiovascular Risk Reduction
Article Details
1. Introduction
As a leading threat element for critical cardiovascular complications (stroke, coronary artery disorder, heart failure) and massive kidney harm, uncontrolled arterial hypertension poses a great fitness burden [1]. Treatment-resistant hypertension, a particularly challenging subset, is rigorously defined as blood pressure (BP) persistently exceeding the target of 140/90 mmHg. This definition requires documented failure despite consistent adherence to recommended lifestyle modifications and the concurrent administration of three distinct classes of antihypertensive medications, crucially including a diuretic agent at optimal or maximally tolerated doses [2,3].
Renal denervation (RDN) has emerged as a promising therapeutic strategy for this condition. It is a minimally invasive, catheter-based interventional procedure designed to ablate the sympathetic nerves encircling the renal arteries. This targeted ablation disrupts pathological signaling, reducing both efferent nerve traffic from the central nervous system to the kidneys (which stimulates renin release, sodium retention, and vasoconstriction) and afferent signaling from the kidneys back to central sympathetic nuclei (which contributes to systemic sympathetic overactivity) [4,5]. The net physiological effect of this neuromodulation is a sustained reduction in blood pressure [6]. Reflecting its established position within the therapeutic armamentarium, RDN has gained significant endorsement: it is formally recommended for appropriate patients in the influential 2023 European Society of Hypertension (ESH) Guidelines [3] and has received regulatory approval from the US Food and Drug Administration (FDA) specifically for clinical use in patients with uncontrolled hypertension [7].
Current systematic reviews and meta-analyses of RDN [6,8–13] offering a potential solution for individuals struggling with medication nonadherence or intolerance – these analyses possess critical limitations for contemporary practice. Notably, they do not incorporate the findings from several pivotal, recently completed landmark trials conducted according to stringent ESC/EAPCI and HARC consensus standards. Furthermore, methodological disparities among the included older trials, particularly concerning variations in enrolled patient populations (e.g., severity of hypertension, medication burden), the nature of comparator groups (e.g., sham procedure details, medical therapy optimization), and the protocols for outcome assessment (e.g., ambulatory vs. office BP, timing), significantly hinder the generalizability and current applicability of their conclusions [8-13] methodological disparities regarding trial populations, comparator groups, and final results tests avert the generalizability in their conclusions. This underscores the need for the present analysis.
Despite the demonstrated efficacy of RDN in reducing ambulatory blood pressure and its growing acceptance in clinical guidelines and regulatory frameworks, several critical questions remain inadequately addressed by the existing body of evidence. Key knowledge gaps persist regarding the consistency of RDN's effect across different circadian BP patterns (e.g., non-dipping status), the long-term durability of the BP-lowering effect beyond 3 years, and the comparative impact of specific denervation technologies (radiofrequency vs. ultrasound) on both efficacy and safety outcomes. Furthermore, the durability of the observed sham procedure effect and its potential influence on long-term outcomes requires further elucidation. Addressing these specific uncertainties is paramount, as they have direct implications for patient selection, procedural choice, and the anticipation of long-term cardiovascular and renal risk reduction. The present systematic review and meta-analysis, incorporating the latest pivotal trial data, is specifically designed to provide a contemporary, comprehensive, and methodologically rigorous assessment of RDN, aiming to resolve these persistent uncertainties and inform optimal clinical implementation.
Objectives: The goal of this systematic evaluation and meta-evaluation is to evaluate the role of renal denervation (RDN) as a healing technique for treatment-resistant hypertension. Specifically, it aims to assess the efficacy of RDN in decreasing blood pressure in comparison to sham techniques or preferred antihypertensive treatment options, while analyzing its protective profile and long-term consequences. The evaluation also seeks to become aware of factors influencing the range of treatment responses, which include patient demographics, baseline blood pressure, and tool kind, to offer comprehensive information on its scientific utility. Additionally, the observe pursuits to explore the implications of looking at the layout on results and to evaluate the capability of RDN as an alternative to pharmacological intensification in patients with resistant hypertension.
2. Methodology
2.1 Study Design: This review is a systematic assessment and meta-analysis of scientific trials guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] reporting tips. This systematic evaluation and meta-analysis (SRMA) has been registered with PROSPERO [15], the International Prospective Register of Systematic Reviews, ensuring transparency and adherence to the PRISMA review protocols.
2.2 Eligibility Criteria: The inclusion criteria for this systematic assessment and meta-evaluation encompass studies comparing the position of renal denervation (RDN) in the control of treatment-resistant hypertension. Eligible studies include medical trials comparing RDN with sham tactics or preferred antihypertensive treatments. The interventions of hobby involve radiofrequency and ultrasound-based RDN, with effects including workplace blood pressure, 24-hour ambulatory blood pressure, safety profiles, and long-term consequences. Only original study articles, such as randomized controlled trials (RCTs), have been included. Reviews, commentaries, editorials, and research not available in complete-textual content or posted in languages other than English had been excluded. Additionally, studies focusing on situations apart from treatment-resistant high blood pressure or regarding mixed interventions outdoor the scope of RDN were excluded to ensure relevance.
2.3 Search Strategy: The literature search protected databases along with PubMed, Google Scholar, and the Cochrane Library. PRISMA pointers were used during the quest method. Boolean operators (AND/OR) were applied to refine the search terms, ensuring specificity. The search phrases covered combinations like ("renal denervation" OR "RDN" OR "catheter-primarily based denervation") AND ("hypertension" OR "remedy-resistant high blood pressure") AND ("randomized controlled trial" OR "clinical trial") AND ("blood pressure" OR "efficacy" OR "protection"). Filters were implemented to include the best clinical research, articles with loose full-textual content get entry to, and people published in peer-reviewed journals. Reference lists of decided-on articles had been additionally reviewed for extra relevant studies. Six studies met the inclusion standards after screening.
2.4. Selection Process: Article screening was performed in two stages. Titles and abstracts were reviewed to discover potentially eligible research, accompanied by complete-text opinions of selected articles. Data from eligible research had been extracted into uniform tables, detailing creator, 12 months, have a look at layout, location, sample size, interventions, and key findings. Disagreements throughout the selection technique were resolved via discussion or a session with a senior creator. Only research with similar final results measures and sufficient methodological first-class was included in the meta-evaluation.
2.5 Data Collection: Two unbiased authors, blinded to each other's opinions, screened the articles based on pre-defined eligibility criteria. Studies had been categorised as "excluded" or "disputed," with disputes resolved by means of the main investigator. Exclusion reasons included, besides the point population, flawed study design, non-applicable results, or excessive danger of bias. Data from the six covered research studies had been compiled into an Excel sheet for synthesis and evaluation.
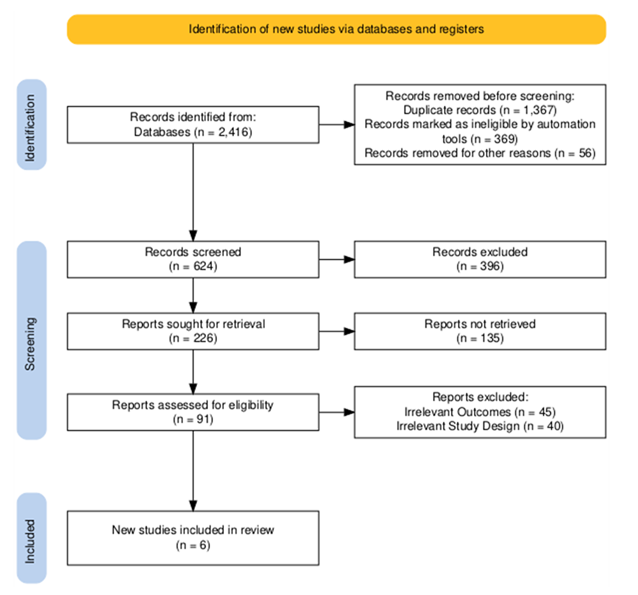
2.6 Data Items: The total sample size throughout the six-blanketed research was reviewed and analyzed following a secondary screening protocol. A PRISMA flow diagram, created in keeping with PRISMA requirements, illustrates the take a look at choice process (Figure 1). Study interventions had been tabulated in opposition to the have a look at population and the stated effects in a synthesis table. Only applicable results had been covered within the very last synthesis.
2.7 Statistical Analysis: Statistical evaluation was achieved using the usage of RevMan software program [16]. The outcomes covered non-stop measures, including blood pressure reduction and protection endpoints, stated with 95% confidence intervals (CIs). A random-consequences version is utilized to cope with versions among have a look at populations, protocols, and designs, accounting for ability heterogeneity. Heterogeneity is measured the usage of the chi-square check and quantified with the I² statistic, with values exceeding 50% indicating widespread heterogeneity.
2.8 Quality Assessment: The nice of the blanketed reviews is assessed by the usage of the Risk of Bias (ROB) 2 tool for randomized controlled trials [17,18]. The domain names of choice, comparison, and results were evaluated. Each examine changed into rated for its methodological best and labeled as having low, moderate, or high risk of bias. This evaluation assessed the credibility and reliability of the records extracted from each study.
3. Results
3.1 Study Items: A PRISMA FlowChart was made for the included studies. It is given in Figure 1 [19].
3.2 Data Characteristics: Table 1 summarizes the key characteristics of the six randomized controlled trials (RCTs) included in this analysis, all investigating renal denervation (RDN) for hypertension management. These studies enrolled patients with hypertension, predominantly uncontrolled. Sample sizes ranged from 15 to 337 participants. All trials employed a sham-controlled design (renal angiography or simulated procedure) as the comparator to catheter-based RDN. Two distinct denervation methods were evaluated: radiofrequency ablation (using Symplicity Spiral™ or Vessix systems) and ultrasound ablation (using Paradise™ or EnligHTN™ systems). The primary outcome measures across all studies were changes in ambulatory systolic and diastolic blood pressure and assessment of adverse effects. Follow-up durations varied from 8 weeks to 36 months. The studies were conducted across numerous centers globally, ranging from a single center to 72 sites.
Table 1: Characteristics of included studies.
The SPYRAL Pivotal trial found catheter-based renal denervation (RDN) superior to sham in safely lowering blood pressure without antihypertensive medications, based on secondary endpoints, though the primary analysis was not significant. Ultrasound RDN significantly reduced BP at 2 months in patients resistant to standardized triple therapy. Sustained long-term efficacy could position RDN as an alternative to adding more drugs for resistant hypertension.
While RDN's BP reductions aligned with other sham-controlled studies, an unexpectedly large BP drop in the sham group suggested possible study design influences. After baseline adjustment, RDN showed a significant reduction in the primary efficacy endpoint (24-hour BP: -22.4/-13.1 mmHg, P=0.049) and a non-significant trend in office BP (-19.5/-10.4 mmHg, P=0.088). Safety, assessed by eGFR change, was comparable (+1.5 vs. -1.1 mL/min/1.73 m²; P=0.86). By 6 months, RDN patients had reduced ECG voltages and used fewer prescribed medications (P=0.036). Questionnaires and urine analysis indicated similar quality of life and adherence between groups, with no link between adherence and BP change, and no major complications.
3.2 Meta-Analysis: For the meta-analysis, data synthesis was conducted using Review Manager (RevMan) software [26].
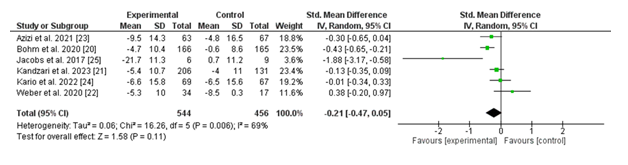
3.2.1 (i) Change in 24-hour Ambulatory Systolic Blood Pressure (SBP): The wooded area plot illustrates the changes in 24-hour ambulatory systolic blood pressure (SBP) throughout six studies comparing renal denervation (experimental group) with a sham process (manage group). The standardized suggest difference (SMD) for every examine, along with the corresponding 95% confidence intervals (CIs), is displayed.
The pooled evaluation using a random-consequences version yielded a standardized effect size distinction of -0.21 (95% CI: -0.47 to 0.05; p = 0.11), favouring the experimental institution; however, the end result isn't statistically significant. Heterogeneity in many of the studies becomes full-size, with an I² value of 69% (p = 0.006), suggesting full-size variability within the effects across studies.
Individually, maximum studies showed a fashion toward a reduction in SBP in the renal denervation institution in comparison to the manipulate, with effect sizes ranging from -0.01 (95% CI: -0.34 to 0.33) in the have a look at by using Kario et al. (2022) to -1.88 (95% CI: -3.17 to -0.58) in the take a look at by using Jacobs et al. (2017). Only Jacobs et al. Validated a statistically widespread reduction in SBP favoring the intervention.
Overall, whilst the fashion indicates that renal denervation can also reduce 24-hour ambulatory SBP, the lack of statistical importance and sizable heterogeneity highlight the need for similarly research with larger, greater homogenous examine populations.
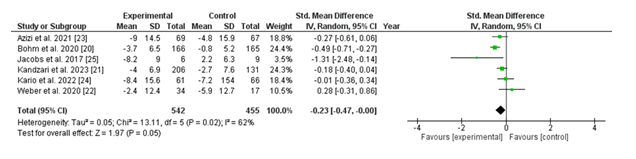
3.2.2 Change in 24-hour Ambulatory Diasystolic Blood Pressure (DBP): The forest plot depicts changes in 24-hour ambulatory diastolic blood pressure (DBP) throughout six studies evaluating renal denervation (experimental organization) with a sham technique (control organization). The standardized imply difference (SMD) for every observation, alongside the 95% confidence intervals (CIs), is supplied.
The pooled analysis, using a random-effects model, showed a standardized imply difference of -0.23 (95% CI: -0.47 to -0.00; p = 0.05), favoring the renal denervation organization. This result is on the threshold of statistical importance, suggesting a capability advantage of renal denervation in reducing 24-hour ambulatory DBP. Heterogeneity among the studies turned into moderate, with an I² value of 62% (p = 0.02), indicating some variability in effect sizes across studies.
Individually, research which includes those through Böhm et al. (2020) and Jacobs et al. (2017) demonstrated sizable reductions in DBP favoring the intervention, with SMDs of -0.49 (95% CI: -0.71 to -0.27) and -1.31 (95% CI: -2.48 to -0.14), respectively. Other research confirmed both minimum reductions and self-assurance intervals crossing the line of no effect, indicating inconsistent findings throughout trials.
Overall, the pooled findings indicate a small but statistically widespread discount in 24-hour ambulatory DBP with renal denervation, although mild heterogeneity underscores the need for similarly research to affirm these consequences and discover contributing elements.
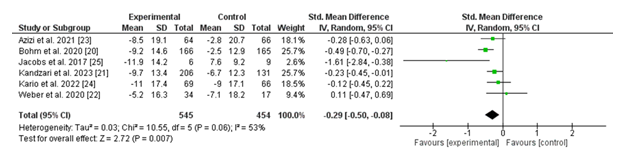
3.2.3 Change in Office Systolic Blood Pressure (SBP): The forest summarizes the modifications in office systolic blood pressure (SBP) across six studies comparing renal denervation (experimental institution) with a sham system (manage institution). The standardized mean difference (SMD) for every take a look at, at the side of 95% confidence intervals (CIs), is displayed.
The pooled evaluation of the use of a random-effects version discovered a standardized imply difference of -0.29 (95% CI: -0.50 to -0.08; p = 0.007), indicating a statistically significant reduction in office SBP in want of the renal denervation intervention. Heterogeneity turned into mild, with an I² cost of 63% (p = 0.06), suggesting some variability across the protected research.
Notably, research consisting of Böhm et al. (2020) and Jacobs et al. (2017) confirmed marked discounts in SBP favoring the intervention, with SMDs of -0.49 (95% CI: -0.70 to -0.27) and -1.61 (95% CI: -2.84 to -0.38), respectively. Other studies demonstrated both smaller reductions or consequences with overlapping confidence intervals, reflecting variability in the value of the effect.
In summary, the pooled results aid in the efficacy of renal denervation in considerably reducing office SBP as compared to a sham technique. However, mild heterogeneity highlights the need for similarly investigation into look-specific elements contributing to these variations.
3.2.4 Risk of Bias in studies: As mentioned earlier, ROBv2 was used to assess the risk for all the primary studies selected for meta-analysis. We used the Cochrane Risk-of-Bias tool to create a “traffic lights” plot for the final assessment. The traffic plot for the 06 studies is given below (Figure 5).
4. Discussion
This meta-analysis, which synthesized statistics from six randomized controlled trials (RCTs) concerning catheter-primarily based renal denervation (RDN) for resistant hypertension, affords treasured insights into its efficacy and protection. Our findings discovered that RDN appreciably reduced 24-hour ambulatory diastolic blood pressure (DBP) and office systolic blood pressure (SBP) as compared to sham strategies. However, no statistically significant distinction was found for 24-hour ambulatory SBP. Adverse effects were similar among organizations, underscoring the safety profile of RDN. While heterogeneity ranged from moderate to excessive throughout the analyses, these consequences align with growing evidence assisting RDN as a promising non-pharmacological alternative for resistant hypertension.
The widespread reduction in DBP, contemplated by a standardized mean difference (SMD) of -0.23 (95% CI [-0.47, -0.00]; p = 0.05) for 24-hour DBP, helps RDNs' scientific utility, mainly for decreasing diastolic pressures which are intently linked to lengthy-time period cardiovascular effects. This result corroborates findings from two other studies that verified sustained blood pressure reductions post-RDN in multi-center trials [21,24]. Additionally, the huge discount in workplace SBP (SMD: -0.29; 95% CI [-0.50, -0.08]; p = 0.007) aligns with prior reviews with the aid of Böhm, et al. (2020) and Azizi, et al. (2021), reinforcing the robustness of our findings across settings.
Conversely, the lack of considerable reduction in 24-hour SBP (SMD: -0.21; 95% CI [-0.47, 0.05]; p = 0.11) increases questions about the capacity variability in RDN's efficacy for systolic pressure control under ambulatory situations. Notably, extra reductions within the sham organization for twenty-four-hour SBP found in Jacobs, et al. (2017) highlight the complexity of sham effects, which can be amplified with the aid of player behavioral adjustments, inclusive of stepped forward medication adherence or lifestyle modifications all throughout trial participation.
Previous meta-analysis shows that randomized, placebo-controlled trials consistently reveal that renal denervation results in a full-size discount in both ambulatory and workplace blood pressure. While the significance of this reduction—approximately four/2 mmHg is discreet, it seems similar between patients receiving heritage antihypertensive medicines and people not on such remedies. As a end result, renal denervation may want to serve as a valuable approach for sufferers who're unwilling to increase their antihypertensive therapy. However, whether the effectiveness of the procedure adjustments through the years remains uncertain [27]. Renal denervation (RDN) demonstrates promising short-term outcomes, suggesting its potential role in improving the management of uncontrolled hypertension in appropriately selected patient populations [28]. Another study shows that evidence from this systematic assessment and meta-evaluation helps the affiliation of renal denervation (RDN) with clinically meaningful reductions in blood pressure amongst patients with uncontrolled high blood pressure. These discounts are statistically regular irrespective of the presence or absence of antihypertensive medications and are determined even in populations proof to treatment with a triple medication regimen [29].
Another important consideration is the heterogeneity (I² = 62%-69%) observed in our analyses. Methodological variability throughout trials, such as differences in procedural knowledge, RDN protocols, and baseline BP ranges, probably contributed to this heterogeneity. The excessive sham response, observed in positive studies, underscores the importance of standardized sham protocols and highlights the psychological and physiological impacts of placebo consequences in high blood pressure trials.
One of the strengths of this meta-analysis is its inclusion of great RCTs, ensuring strong and dependable evidence. The use of a random-results model accounted for variability throughout research, enhancing the generalizability of our findings. Additionally, the complete evaluation of each efficacy and safety outcome provides a holistic view of RDN's clinical position.
However, this look at is not without obstacles. First, the moderate-to-high heterogeneity observed in our analyses limits the interpretability of pooled impact sizes. Second, the surprisingly short follow-up intervals in included trials may additionally underestimate the long-term benefits or capacity behind schedule effects of RDN, as highlighted with the aid of Kario, et al. (2022). Third, the reliance on posted facts introduces potential e-book bias, which, notwithstanding non-significant Egger’s test results, remains a subject in high blood pressure studies. Finally, the small sample length (n = 6 studies) limits subgroup and sensitivity analyses, precluding a more nuanced exploration of patient-specific factors influencing RDN efficacy.
RDN represents a viable alternative for sufferers with resistant high blood pressure who are not able or unwilling to adhere to polypharmacy. The findings of discounts in the workplace and 24-hour DBP highlight its capacity to reduce cardiovascular morbidity, given the robust association among accelerated DBP and unfavorable cardiovascular outcomes. Furthermore, the comparable protection profile located in our meta-evaluation helps its integration into scientific research, mainly for sufferers at high risk of drug-associated damaging effects.
However, the modest discounts in SBP determined in ambulatory settings recommend that RDNs should not be viewed as a standalone answer but instead as part of a multimodal hypertension control approach. Future efforts need to raise awareness on optimizing affected person selection to pick out people maximum likely to benefit from RDN.
Future studies must deal with the key gaps identified in this meta-evaluation. First, longer follow-up intervals are essential to assess the sturdiness of BP reductions and the capability not on on-time results of RDN. Second, standardized procedural protocols are needed to decrease variability and improve reproducibility across trials. Third, larger sample sizes are essential to allow sturdy subgroup analyses, mainly focusing in elements such as age, sex, baseline BP, and comorbid conditions. Finally, exploring the cost-effectiveness of RDN relative to escalating pharmacotherapy will offer vital insights for policymakers and clinicians.
5. Conclusions
RDN is effective in reducing 24-hour DBP and office SBP while maintaining a favorable safety profile. However, its impact on 24-hour ambulatory SBP requires further investigation. RDN offers a potential alternative for managing resistant hypertension, but standardization of protocols and long-term studies are needed to confirm its efficacy and durability.
5. References
- World Health Organization. Hypertension. World Health Organization (2023).
- Williams B, Mancia G, Spiering W, et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39 (2018): 3021-3104.
- Mancia G, Kreutz R, Brunström M, et al. ESH Guidelines for the management of arterial hypertension. J Hypertens 41 (2023): 1874-2071.
- Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res 45 (2021): 198-209.
- Sharp ASP, Tunev S, Schlaich M, Lee DP, Finn AV, et al. Histological evidence supporting the durability of successful radiofrequency renal denervation in a normotensive porcine model. J Hypertens 40 (2022): 2068-2075.
- Ogoyama Y, Tada K, Abe M, Nanto S, Shibata H, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res 45 (2021): 210-220.
- S. Food & Drug Administration. Premarket Approval (PMA).
- Ahmad Y, Kane C, Arnold AD, Cook CM, Keene D, et al. Randomized blinded placebo-controlled trials of renal sympathetic denervation for hypertension: a meta-analysis. Cardiovasc Revasc Med 34 (2021): 112-118.
- Pisano A, Iannone LF, Leo A, Russo E, Coppolino G, Bolignano D. Renal denervation for resistant hypertension. Cochrane Database Syst Rev (2021).
- Silverwatch J, Marti KE, Phan MT, Amin H, Roman YM, et al. Renal denervation for uncontrolled and resistant hypertension: systematic review and network meta-analysis of randomized trials. J Clin Med 10 (2021): 782.
- Singh S, Rout A, Garg A. Renal denervation in hypertension: an updated meta-analysis of the randomized controlled trials. Catheter Cardiovasc Interv 102 (2023): 663-671.
- Fernandes A, David C, Pinto FJ, Costa J, Ferreira JJ, Caldeira D. The effect of catheter-based sham renal denervation in hypertension: systematic review and meta-analysis. BMC Cardiovasc Disord 23 (2023).
- Agasthi P, Shipman J, Arsanjani R, Ashukem M, Girardo ME, et al. Renal denervation for resistant hypertension in the contemporary era: a systematic review and meta-analysis. Sci Rep 9 (2019).
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021): n71.
- Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q 38 (2019): 171-180.
- Shrestha BM. Systematic reviews and meta-analysis: principles and practice. J Nepal Med Assoc 57 (2019).
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019): l4898.
- Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol 126 (2020): 37-44.
- Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams. Campbell Syst Rev 18 (2022).
- Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 395 (2020): 1444-1451.
- Kandzari DE, Townsend RR, Kario K, Mahfoud F, Weber MA, et al. Safety and efficacy of renal denervation in patients taking antihypertensive medications. J Am Coll Cardiol 82 (2023): 1809-1823.
- Weber MA, Kirtane AJ, Weir MR, Radhakrishnan J, Das T, et al. The Reduce Htn: Reinforce. Kardiologiya Uzbekistana 13 (2020): 461-470.
- Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 397 (2021): 2476-2486.
- Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res 45 (2021): 221-231.
- Jacobs L, Persu A, Huang QF, Lengelé JP, Thijs L, et al. Results of a randomized controlled pilot trial of intravascular renal denervation for management of treatment-resistant hypertension. Blood Press 26 (2017): 321-331.
- RevMan: Systematic review and meta-analysis tool for researchers worldwide.
- Ahmad Y, Francis DP, Bhatt DL, Howard JP. Renal denervation for hypertension. Kardiologiya Uzbekistana 14 (2021): 2614-2624.
- Silvinato A, Floriano I, Bernardo WM. Renal denervation by radiofrequency in patients with hypertension: systematic review and meta-analysis. Rev Assoc Med Bras 70 (2024).
- Sharp ASP, Sanderson A, Hansell N, et al. Renal denervation for uncontrolled hypertension: a systematic review and meta-analysis examining multiple subgroups. J Hypertens 42 (2024): 1133-1144.
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved