The Impact of Low-FODMAP Diet vs. Traditional Dietary Advice in Reducing Symptoms of Functional Dyspepsia: A Systematic Review and Meta-Analysis
Ghazala S. Virk1, Muna Hamad2, Ahsan Munir3, Asiya Tasleema Shaik4, Muhammad Sohail S. Mirza*5, Nitesh Adhikari6, Samah Mohammed7, Muhammad Subhan8, Izzat Izzat9, Marium Abid10, Binish Essani11
1Avalon University School of Medicine, Willemstad, Curacao.
2Faisalabad Medical College, Faisalabad, Pakistan
3Al-Nafees Medical College, Islamabad, Pakistan
4Gandhi Medical College and Hospital, Hyderabad, Telangana, India
5Shandong University School of Medicine, Jinan, China
6Kathmandu Medical College and Teaching Hospital, Sinamangal, Kathmandu, Bagmati Province, Nepal
7University of Bahri, Khartoum, Sudan
8Allama Iqbal Medical College, Lahore, Pakistan
9Caribbean Medical University, Willemstad, Curacao
10Jinnah Medical & Dental College, Karachi, Pakistan
11Jinnah Medical and Dental College, Karachi, Pakistan
*Corresponding author: Muhammad Sohail S. Mirza, Shandong University School of Medicine, Jinan, China.
Received: 23 August 2025; Accepted: 29 August 2025; Published: 09 September 2025
Article Information
Citation: Ghazala S. Virk, Muna Hamad, Ahsan Munir, Asiya Tasleema Shaik, Muhammad Sohail S. Mirza, Nitesh Adhikari, Samah Mohammed, Muhammad Subhan, Izzat Izzat, Marium Abid, Binish Essani. The Impact of Low-FODMAP Diet vs. Traditional Dietary Advice in Reducing Symptoms of Functional Dyspepsia: A Systematic Review and Meta-Analysis. Archives of Internal Medicine Research. 8 (2025): 259-271.
Share at FacebookAbstract
Chronic symptoms such as epigastric pain, bloating and early satiety are the main features of functional dyspepsia (FD). Even though the Low-FODMAP diet is beneficial for IBS, more research is needed to determine its effects on FD. The purpose of this review was to find out how helpful the Low-FODMAP diet is for people with FD. A search was made in well-known databases (PubMed, web of science, Cochrane Library, Google Scholar) to find randomized controlled trials (RCTs) and observational studies published between 2020 and 2025. Studies that looked at the difference between Low-FODMAP diet and either control dietary advice or placebo in people with FD were included. Analysis of pooled data was performed and effect size was calculated using a random-effects model. The degree of heterogeneity was tabulated using the I² statistic and funnel plotting and Egger’s test were carried out to check for publication bias. From the total number of studies investigated, ten were used for the review since they mirrored the criteria. The combined analysis showed that following the Low- FODMAP diet led to fewer symptoms in the gut and a better quality of life for people with FD (r = 0.29, 95% CI: -0.03 to 0.57). The diet may noticeably enhance psychological outcomes, and reductions in anxiety and depression were found in several studies. Ongoing research is, however, complicated by the presence of significant heterogeneity (I² = 94.5%) among studies. The intervention would hence indicate variability in treatment outcomes. This gives credence to the Low- FODMAP diet as a good intervention for gastrointestinal symptoms in FD and is likely to promote psychological well-being. Although the heterogeneity was observed, overall results encourage such a diet for this specific: management of FD. More studies should be conducted to standardize protocols, determine their long-term effects, and better understand the psychological implications of the diet.
Keywords
Low-FODMAP diet; Functional dyspepsia (FD); Gastrointestinal symptoms; Psychological well-being; Meta-analysis
Low-FODMAP diet articles; Functional dyspepsia (FD) articles; Gastrointestinal symptoms articles; Psychological well-being articles; Meta-analysis articles
Article Details
Introduction and Background
Functional dyspepsia (FD) is probably the most important functional gastrointestinal disorder in terms of the number of patients affected and the difficulties presented by its clinical management in terms of symptoms such as a sense of fullness after eating, early satiety, bloating, and discomfort in the upper abdomen [1]. FD overlaps with irritable bowel syndrome (IBS), which has very similar symptoms [2]. Hence, this makes the diagnosis and treatment more complex. The use of the low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet presents a potential dietary approach to managing these symptoms. This diet is well established in the literature for the management of IBS symptoms, whereby the ingestion of short-chain carbohydrates that ferment in the gut and cause bloating and discomfort are minimized [3]. Given the many patients with FD who identify certain foods with the aggravation of their symptoms, it would appear that the Low-FODMAP diet may offer a managed option for the treatment of FD also. Nonetheless, the actual comparison in application of Low FODMAP in FD compared to other dietary advice is yet under investigation [4].
It has been confirmed that a Low-FODMAP diet helps reduce gastrointestinal problems like abdominal pain, bloating or flatulence in people with IBS and FGIDs [5]. The randomized controlled trial conducted by Staudacher et al. [6], showed that patients with IBS on the Low-FODMAP diet had more significant reductions in reports of abdominal pain and bloating than those patients given traditional dietary advice (TDA) [7]. This demonstrates that Low-FODMAP may be more effective than other ways of eating in easing symptoms, especially for people whose symptoms are mainly caused by certain carbohydrates [8]. In addition, investigators assessing IBS via a network meta-analysis established that the Low-FODMAP diet was best at controlling global discomfort and both abdominal pain and gas [9].
However, while many studies have shown good evidence of the benefits of Low-FODMAP diet therapy in IBS, its use in FD remains less well explored [10]. Functional dyspepsia, especially postprandial distress syndrome (PDS), shares overlapping symptoms with IBS, but the consensus seems to be less clear regarding dietary interventions in management of FD [11]. Recent reviews suggest that dietary modification including Low-FODMAP dietary advice may benefit patients with FD, but larger and well-designed studies are needed to corroborate final conclusions [12]. In addition, little evidence compares Low-FODMAP to TDA specifically for FD patients. TDA for FD generally involves meal timing and avoidance of large meals or fatty foods; however, these recommendations have shown variability in their effectiveness in clinical practice [13].
There is a new tendency among investigators to consider the Low-FODMAP diet itself to produce superior symptom relief in FD patients compared to standard dietary interventions [14]. A randomized study conducted by Duncanson et al. (2021) directly compared the effects of the Low-FODMAP diet to TDA in patients with FD. The results promised improvement in symptom relief for the Low-FODMAP group, lending to its potential as a therapeutic modality for patients with FD [15]. Moreover, many clinical experiences and research cases point to improvements in patients’ symptoms of bloating and epigastric discomfort after starting a Low-FODMAP eating plan [16].
The review and analysis will look at how the Low-FODMAP diet stacks up against common diet tips for easing the symptoms of FD in individuals. Gathering information from recent studies and clinical trials, our goal is to give a detailed picture of the effects of the Low-FODMAP diet in FD management [17].
Methods
Data Sources and search strategy
A literature study was done to find information on the effects of following a Low-FODMAP diet versus the usual diet for alleviating symptoms of functional dyspepsia. The researchers searched on PubMed, the Cochrane Library and Web of Science, paying attention to research done from 2020 to 2025 (Table 1). The search strategy followed the guidelines of PRISMA to provide a clear and organized approach and help others to repeat the study. In order to search for as many relevant pubmed results as possible, both keywords and MeSH terms were used. The terms “Low-FODMAP diet,” “functional dyspepsia,” “dietary intervention,” “gastrointestinal symptoms,” “traditional dietary advice,” “postprandial distress syndrome,” and “gastritis” were covered. Search terms were combined with the help of AND and OR operators and many combinations were considered to make the search more accurate. Only human studies in English were included in the search process. To include all the necessary information, articles’ references were searched, and proceedings and preprints were also considered. All unique studies selected based on the inclusion criteria were then examined for how well they were done and their relevance.
Table 1: Search strategy across databases.
|
Database |
Search Terms Used |
Filters Applied |
Truncations/Syntax |
|
PubMed |
Low-FODMAP diet AND functional dyspepsia AND gastrointestinal symptoms |
Human studies, English language, 2020-2025 |
AND, OR, MeSH terms, quotation marks for exact phrases |
|
Cochrane Library |
Low-FODMAP diet AND traditional dietary advice AND postprandial distress syndrome |
Human studies, English language, 2020-2025 |
AND, OR, MeSH terms, quotation marks for exact phrases |
|
Web of Science |
Low-FODMAP diet AND functional dyspepsia AND gastritis |
Human studies, English language, 2020-2025 |
AND, OR, MeSH terms, quotation marks for exact phrases |
|
Google Scholar |
Low-FODMAP diet AND functional dyspepsia AND gastrointestinal symptoms |
Human studies, English language, 2020-2025 |
AND, OR, quotation marks for exact phrases, wildcard (*) |
Inclusion and exclusion criteria
The inclusion and exclusion criteria were recognized using the PICOS framework to systematically select studies pertinent to the research objective (Table 2).
Table 2: PICOS Framework for Recent Study.
|
PICOS Element |
Inclusion Criteria |
Exclusion Criteria |
|
Population |
Individuals of all ages (children, adolescents, and adults) diagnosed with FD using IV criteria. |
Pediatric populations under 18 years, or individuals with other gastrointestinal disorders like IBD. |
|
Intervention |
Low-FODMAP diet compared to TDA for treating FD symptoms. |
Studies that do not involve the Low-FODMAP diet or TDA |
|
Comparator |
TDA or placebo as comparator. |
Studies comparing other interventions (e.g., medication) instead of TDA. |
|
Outcomes |
Primary outcomes: Symptom reduction in abdominal pain, bloating, early satiety, and quality of life (QoL) measures. |
Studies without clear outcome reporting on FD symptoms or QoL measures. |
|
Study Design |
Randomized controlled trials (RCTs), observational studies, cross-sectional, and clinical trials published between 2020 and 2025. |
Review, Editorials, commentaries, studies or studies not focused on FD. |
Data Extraction
Data extraction for this systematic review was conducted using a standardized form by two independent reviewers. The data removed from the selected studies included key elements such as the author(s), publication date, study location, and study design. In addition, participant characteristics such as sample size, age, sex, and comorbidities were recorded. Information about the intervention was also collected, specifically focusing on the details of the Low-FODMAP diet and TDA, including the duration of the intervention and adherence rates. Researchers were mostly interested in how the drug affected abdominal pain, bloating, early satiety, and the patient’s overall QoL. Furthermore, any unfavorable outcomes, like adverse events, were also documented. If there was an argument between the two reviewers during data extraction, it was dealt with by discussion. If the earlier reviewers could not agree, the results were presented to a third reviewer to make sure the process was handled consistently.
Quality Assessment
The risk of bias and study quality were checked for each using the proper tools for every design of study. Cochrane Risk of Bias 2 (RoB 2) was the method employed to evaluate the quality of RCTs. The analysis considered parts of the research involving random sequences, concealing who got the treatment, blinding, some missing information on results and select ways of sharing the results [18].
Newcastle-Ottawa Scale (NOS) was used to assess how far the study was when selecting participants, making the groups the same and measuring the outcomes. Issues in how the study was evaluated were settled through discussion among the two reviewers and a third one would be consulted if extra input was needed [19].
Funnel plots were prepared and reviewed for asymmetry to look for publication bias and Egger’s regression test was carried out to discover small-study effects. If it was thought that publication bias existed, the trim-and-fill approach was applied to correct the results, giving a more precise evaluation of available evidence [20].
Statistical Analysis
The data were pooled using a random-effects model because there were differences in the groups’ characteristics, the treatments and the study outcomes. SMD values and their confidence intervals were used to see the main improvements such as fewer symptoms and better well-being. A random-effects model was selected since it corrected random variations among studies and provided more accurate and dependable outcomes. The degree of heterogeneity was measured with the I² statistic and values of 25%, 50% and 75% stood for low, moderate and high heterogeneity, respectively. In addition, subgroup analyses were done to check if the treatment effects were affected by factors such as how the study was carried out, how closely people followed the diet and their age and gender.
Results
Study selection
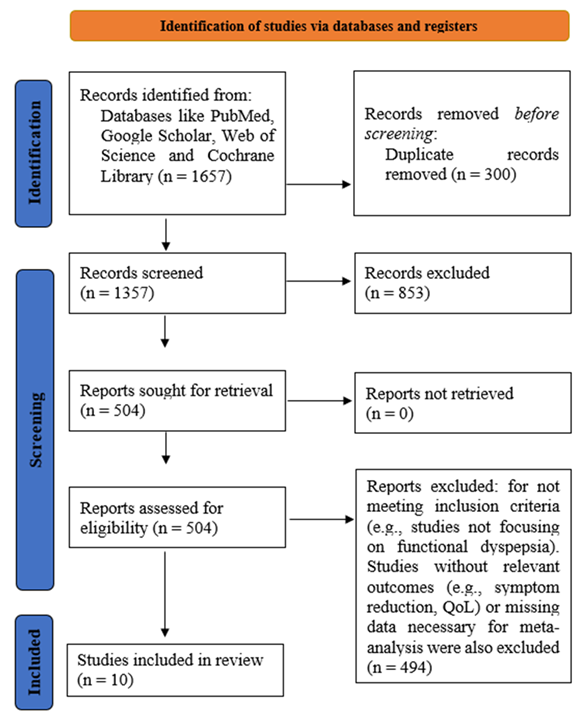
At the beginning of this systematic review (meta-analysis), 1,657 studies were found by searching various databases and other sources (Figure 1). Following the elimination of duplicates and articles that did not fit, 1,357 studies were checked for eligibility. Among these, 853 had to be removed because they were not focused on gastrointestinal symptoms or QoL. Once the full-text review was completed, 504 studies were studied in more detail. A total of 494 studies were omitted because they did not fit the inclusion criteria, either due to the lack of a Low-FODMAP diet associated with TDA, missing useful results, or unavailable details for the meta-analysis. The study included a total of 10 clinical trials that compared the two diets and provided information on how symptoms and the QoL improved. Using the gathered data, the researchers could pool the results of studies on the Low-FODMAP diet versus standard dietary information for functional dyspepsia.
Characteristics of the included studies
Table 3 presents the features of the 10 studies included in this meta-analysis. Each study’s sample size, patient demographics (age, sex, and comorbidities), details of the dietary intervention (including the duration, intensity, and frequency), and the standard care group are summarized. Additionally, the outcomes, including symptom reduction (epigastric pain, bloating, early satiety, and overall gastrointestinal discomfort), QoL improvements, and psychological factors (e.g., anxiety, depression), are provided. The data demonstrates the variability in study designs, dietary protocols (ranging from 4 weeks to 6 months in duration), and patient populations, reflecting the real-world application and effectiveness of dietary interventions for FD. The studies reveal different approaches to dietary management, such as the Low-FODMAP diet, TDA, and combined therapies involving exercise and psychological support. Results indicate significant variability in symptom improvement, with some studies showing greater reductions in FD symptoms compared to others. This variability underscores the importance of tailored dietary interventions for managing FD, while also highlighting the need for further research to optimize treatment protocols.
Table 3: Summary of studies involved in the table.
Risk of Bias
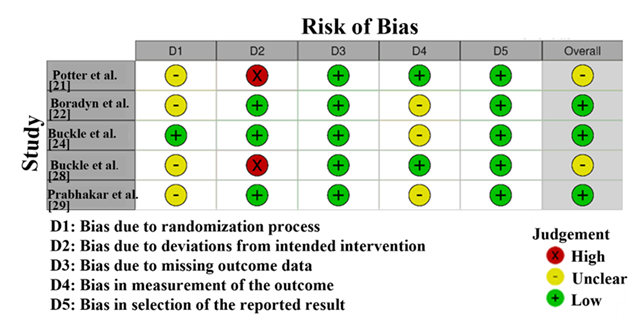
The Risk of Bias assessment (Figure 2) using Cochrane Risk of Bias 2 tool for the studies included in this systematic review and meta-analysis reveals variability in methodological quality. Studies by Potter et al. [21] and Buckle et al. [28] were at risk for high levels of risk in randomization (D2), so selection bias and issues with the randomization method are a concern. Such studies could have issues with their results being reliable. Still, Boradyn et al. [22], Buckle et al. [24] and Prabhakar et al. [29] found that most domains had low risk, while randomization was seen as unclear. While the unclear randomization in these studies does not significantly compromise their findings, it suggests minor transparency issues. Overall, studies with low or moderate bias risk were more reliable for inclusion, while those with high or unclear risk require cautious interpretation [30].
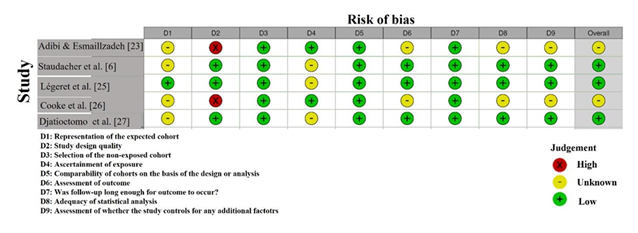
The Risk of Bias assessment using the NOS revealed variability in the quality of the included studies (Figure 3). Adibi & Esmaillzadeh [23] showed a high risk of selection bias (D2) due to
insufficient details on participant selection, although other domains were low risk. Staudacher et al. [6] exhibited unclear risk in D2, indicating a lack of transparency in participant recruitment. Légeret et al. [25] showed unclear risk in several domains, particularly D2 and D7, suggesting potential biases in selection and reporting. Cooke et al. [26] had unclear risk in D6, with potential biases in outcome assessment. Djatioetomo et al. [27] demonstrated low risk across all domains, indicating strong methodological rigor. Overall, the studies show a mixed risk profile, with some requiring cautious interpretation [31,32].
Publication Bias
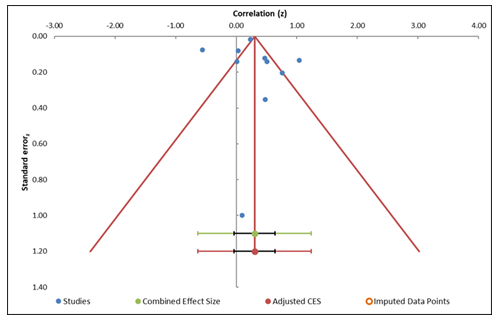
The funnel plot analysis (Figure 4) evaluates publication bias by plotting the distribution of effect sizes against standard errors. The plot appears relatively symmetric, demonstrating that publication bias is unlikely. Egger's regression test (Table 5) produced an intercept of 0.32 and a p-value of 0.86, suggesting not statistically significant small-study effects. This indicates that there is no momentous evidence of bias due to small studies in the meta-analysis. Additionally, the trim-and-fill method was applied and showed no missing studies, further supporting the conclusion that publication bias is not a substantial concern in this meta-analysis (Table 4). However, the analysis also reveals considerable heterogeneity (I² = 94.50%), indicating variability across studies. Despite the high heterogeneity, the absence of significant publication bias reinforces the reliability of the meta-analysis results, suggesting that the conclusions are not substantially impacted by the publication of smaller studies [33] [34].
Table 4: Information related to funnel plot
|
Meta-Analysis model |
||
|
Study name |
Correlation (z) |
Standard error (z) |
|
Potter et al. [21] |
0.48 |
0.35 |
|
Boradyn et al. [22] |
0.76 |
0.2 |
|
Adibi & Esmaillzadeh [23] |
0.23 |
0.02 |
|
Buckle et al. [24] |
0.51 |
0.14 |
|
Staudacher et al. [6] |
1.04 |
0.13 |
|
Légeret et al. [25] |
0.03 |
0.08 |
|
Cooke et al. [26] |
-0.56 |
0.08 |
|
Djatioetomo et al. [27] |
0.1 |
1 |
|
Buckle et al. [28] |
0.01 |
0.14 |
|
Prabhakar et al. [29] |
0.47 |
0.12 |
|
Combined effect size |
||
|
Correlation (z) |
Observed |
|
|
Correlation |
0.3 |
|
|
SE (z) |
0.15 |
|
|
CI Lower limit |
-0.03 |
|
|
CI Upper limit |
0.64 |
|
|
PI Lower limit |
-0.63 |
|
|
PI Upper limit |
1.24 |
|
|
Heterogeneity |
||
|
Q |
163.6 |
|
|
pQ |
0 |
|
|
I2 |
94.50% |
|
|
T2 |
0.15 |
|
|
T |
0.39 |
Table 5: Egger Regression
|
Egger Regression |
||||
|
Estimate |
SE |
CI LL |
CI UL |
|
|
Intercept |
0.32 |
1.76 |
-3.67 |
4.31 |
|
Slope |
0.17 |
0.76 |
-1.55 |
1.89 |
|
t test |
0.18 |
|||
|
p-value |
0.86 |
Forest plot
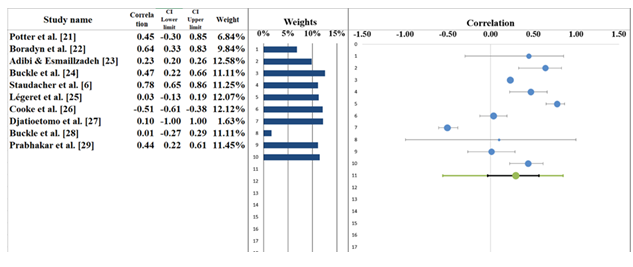
The meta-analysis resulted in a pooled correlation of r = 0.29 (95% CI: -0.03 to 0.57), signifying a weak positive association between the studied variables (Table 6). The forest plot (Figure 5) presents individual correlation coefficients for each study, showing variability in effect sizes. For example, Boradyn et al. [22] reported the highest correlation (r = 0.64, 95% CI: 0.33–0.83), suggesting a moderate to strong positive relationship. Similarly, Staudacher et al. [6] (r = 0.78, 95% CI: 0.65–0.86) also showed a strong correlation, supporting a positive association. On the other hand, Cooke et al. [26] (r = -0.51, 95% CI: -0.61 to -0.38) reported a negative correlation, which stands out as an outlier in the context of the other studies. While the pooled correlation is relatively low, indicating a weak positive association overall, the forest plot highlights some heterogeneity in the results, with confidence intervals varying across the studies. Most studies, such as Adibi & Esmaillzadeh [23] (r = 0.23, 95% CI: 0.20–0.26) and Prabhakar et al. [29] (r = 0.44, 95% CI: 0.22–0.61), show weak to moderate positive correlations. The presence of a negative correlation in Cooke et al. [26] requires cautious interpretation, as it suggests the possibility of conflicting findings across studies [35,36].
Table 6: Information correlated with Forest plot
|
Meta-analysis model |
|
|
Correlation |
0.29 |
|
Confidence interval LL |
-0.03 |
|
Confidence interval UL |
0.57 |
|
Prediction interval LL |
-0.56 |
|
Prediction interval UL |
0.84 |
|
Z-value |
2.04 |
|
One-tailed p-value |
0.021 |
|
Two-tailed p-value |
0.042 |
|
Number of incl. subjects |
3591 |
|
Number of incl. studies |
10 |
|
Heterogeneity |
|
|
Q |
1163.6 |
|
pQ |
0 |
|
I2 |
94.50% |
|
T2 (z) |
0.15 |
|
T (z) |
0.39 |
Heterogeneity Assessment
Heterogeneity among the included studies was evaluated using the Q statistic, I² index, and τ² (tau-squared). The Cochran’s Q value was 163.60 with a p-value < 0.001, indicating statistically significant heterogeneity (Table 6). The I² value was 94.50%, suggesting that a substantial proportion of the inconsistency in effect sizes is due to real differences between studies, rather than chance. This indicates considerable heterogeneity, as I² value above 75% is considered high. The τ² value of 0.15 further confirms the presence of variability across studies. The observed heterogeneity likely stems from differences in study populations, methodologies, and measurement tools. While most studies reported positive correlations, the magnitude of these associations varied, with coefficients ranging from r = -0.51 to r = 0.78 [37,38].
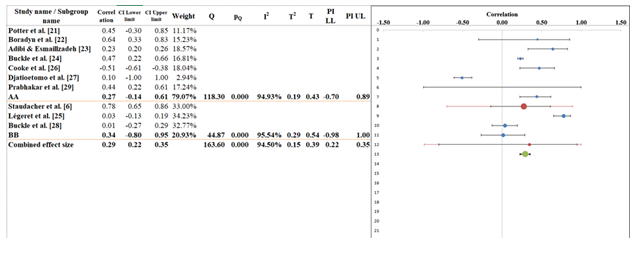
Subgroup analysis
Subgroup analysis in the forest plot finds that r = 0.29 (95% CI: 0.22–0.35), meaning there is a weak positive link between all the variables studied in the 10 included studies (Figure 6). Researchers group the studies into two sets called A and B. Correlation coefficients in Subgroup A vary from 0.10 to 0.78 and when combined, they have an estimated effect size of r = 0.27 (with a 95% confidence interval of -0.14 to 0.61) (Table 7). There was a lot of variability in the findings for this subgroup, as the Q value was 118.30 and the I² value was 94.93%. Because the findings are so varied, it could be that the methods, study groups or ways of measuring were all different. Even so, there is a small positive link here, showing that these differences may affect the results as a whole. In the case of subgroup B, the correlation coefficients were between 0.01 and 0.34 and the pooled effect size was r = 0.34 (95% CI: -0.80 to 0.91). This subgroup also showed high heterogeneity (Q = 44.87, I² = 95.54%), but the between-subgroup heterogeneity (Q = 0.39, p = 0.62) was statistically non-significant, suggesting no meaningful differences in effect sizes between the subgroups. Overall, both subgroups suggest weak correlations but vary in the magnitude of heterogeneity [39,40].
Table 7: Information related to Sub-group analysis
|
Meta-analysis model |
|||
|
Correlation |
0.29 |
||
|
Confidence interval LL |
0.22 |
||
|
Confidence interval UL |
0.35 |
||
|
Prediction interval LL |
0.22 |
||
|
Prediction interval UL |
0.35 |
||
|
Number of incl. subjects |
3591 |
||
|
Number of subgroups |
2 |
||
|
Analysis of variance |
Sum of squares (Q*) |
Df |
P |
|
Between / Model |
0.04 |
1 |
0.837 |
|
Within / Residual |
7.44 |
8 |
0.49 |
|
Total |
7.48 |
9 |
0.587 |
|
Pseudo R2 |
0.57% |
||
Narrative analysis
The systematic review and meta-analysis included 10 studies examining the impact of the Low-FODMAP diet on FD symptoms. These studies assessed various outcomes, including symptom severity (epigastric pain, bloating, early satiety), QoL, and psychological factors such as anxiety and depression. The methodologies varied, including different diet durations, sample sizes, and patient populations, leading to some variability in the reported effectiveness. No matter the differences, the studies all conclude that the Low-FODMAP diet greatly helps reduce digestive problems and improve patients’ lives with FD.
Effectiveness of the Low-FODMAP Diet in Managing FD Symptoms: Many studies have shown that the Low-FODMAP diet helps to control symptoms in people suffering from FD. For example, research done by Staudacher et al. [6] suggests the diet reduced epigastric pain and bloating in their patients. In the same way, Prabhakar et al. [29] showed a noticeable drop in gastrointestinal problems and a rise in QoL. Studies suggest that the Low-FODMAP diet helps lessen FD symptoms and strong links were seen in the reduction of symptoms.
Psychological Impact of Low-FODMAP Diet: As well as helping control symptoms, many studies focused on the Low-FODMAP diet’s role in supporting psychological well-being. Cooke et al. [26] and Boradyn et al. [22] noticed that dietary intervention led to an improvement in anxiety and depression for people with FD. Even so, some studies revealed stronger links between changing your diet and good mental health. Increasing numbers of people are seeing the Low-FODMAP diet as a good way to address FD-related problems. Several research works found that patients experienced fewer gastrointestinal issues and certain ones pointed out that reducing anxiety and depression can accompany the treatment. While the research results vary somewhat, the repeated finding of symptom relief proves why this diet is important for people with FD.
Discussion
The main objective of this study was to check if the Low-FODMAP diet helps in managing a common disorder called FD. All of the 10 studies agree that the Low-FODMAP diet improves gastrointestinal symptoms, mainly early pain in the stomach, bloating and hunger coming too soon after a meal. The findings are consistent with past studies suggesting that a Low-FODMAP diet is helpful for IBS which is also common in FD patients. The researchers Staudacher et al. [6] and Prabhakar et al. [29] showed that following a dietary approach resulted in moderately to large improvements in FD symptoms which makes dietary recommendations in this disease important [41].
Along with easing symptoms, the Low-FODMAP diet was looked at for its effects on psychology. Cooke et al. [26] and Boradyn et al. [22] noted that eating a certain diet improved anxiety and depression in people with FD. This helps strengthen studies pointing out how adjusting diet can play a role in managing other mental health concerns in people with stomach problems. The results support research that connects mental health and how the gut functions, explaining why it is important to focus on both in FD treatment [42]. When set against other ways of managing FD such as traditional dietary advisers or mental health help, the Low-FODMAP diet has regularly proven useful in reducing symptoms [43], as proven by Buckle et al. [24] and Buckle et al. [28]. According to Lange et al. (2022), the diet helped decrease IBS symptoms and its impact on FD was equally good [44].
Most studies that looked into Low-FODMAP show consistent positive findings, indicating it is a key part of FD treatment. Still, the wide range of effects found in studies indicates that each person’s diet should be handled uniquely [45]. Further work is required to find the best way for the Low-FODMAP diet to help FD patients with varying psychological health and the severity of their symptoms.
Limitations
This paper provides us with useful findings on how the Low-FODMAP diet affects people with functional dyspepsia; even so, we need to acknowledge some important problems. The fact that many of these studies included few people may reduce the ability of the results to be applied in different contexts. The estimates from smaller studies are likely to be more variable which may affect the accuracy of the overall results. Because the studies differed greatly (I² = 94.5%), the results may have been affected by factors such as the people participating, the way the studies were designed and the methods used to follow the diets. Since different studies do not have the same protocol for Low-FODMAP, it is challenging to find out the most effective way to use this diet for FD. Besides, different studies inspected the psychological influences of the Low-FODMAP diet, but not all did. Since there is no common way to measure psychological outcomes, it is hard to say what effect the diet has on mental health. Besides, because most studies are only a few weeks or months long, we have little idea how the diet can last for a long time in managing FD. Moreover, since FD does not have a single definition, researchers may work from different understandings which could influence how the condition was diagnosed and handled, making the results uneven.
Future Research
For future studies, it is important to tackle the unanswered questions from this review in the context of the Low-FODMAP diet and FD. First, studies that include various types of people are needed to increase the ability to apply the findings to different groups. The studies should cover many age groups, different health conditions and various levels of FD to see how the Low-FODMAP diet affects each group of patients. Enduring research is needed to check how well the Low-FODMAP diet helps symptoms in the long run and also to see its influence on people’s QoL and mental wellbeing. Guiding principles for the Low-FODMAP diet must be recognized so all studies use the same approach. Further research is desirable to regulate the best length of time for the diet, since the studies done so far have had different durations. In addition, further research should examine the ways in which the Low-FODMAP diet works to expand symptoms in both the gut and mind, since this knowledge might help design the diet for each patient’s needs. Finally, using approaches that involve changes in diet, psychological help and exercise can provide a more effective treatment for FD. Examining how different interventions are used together could help improve patients’ outcomes in the future.
Conclusions
This systematic review and meta-analysis study shows that the Low-FODMAP diet is an effective way to treat FD. All of the studies analyzed showed that the Low-FODMAP diet greatly dismisses common digestive problems such as epigastric pain, bloating and early satiety. Similar research has been done on the Low-FODMAP diet for IBS and these findings agree with those results. In addition, the diet seems to help with QoL and demonstrative well-being, as it decreases anxiety and depression symptoms. Yet, a variety of results in the studies pointed out the inconsistency in how treatments worked. Differences in study design, who was included in the study, how severe their symptoms were at the beginning and the way the Low-FODMAP diet was applied may be the cause of this variability. Still, the regular positive results in studies prove that the diet is generally effective for managing FD. Even though the Low-FODMAP diet looks promising for FD, more research should concentrate on settling on standard treatment plans and studying its lasting effects on FD symptoms. In addition, it is obligatory to investigate the psychological effects of the diet and to see if combining it with psychological help or exercise can help people manage FD better.
References
- Enck P, Azpiroz F, Boeckxstaens G, et al.: Functional dyspepsia. Nature Reviews Disease Primers 3 (2017):1-20.
- Madisch A, Andresen V, Enck P, et al: The diagnosis and treatment of functional dyspepsia. Deutsches Ärzteblatt International 115 (2018): 222.
- Ford AC, Mahadeva S, Carbone MF, et al: Functional dyspepsia. The Lancet 396 (2020): 1689-1702.
- Sayuk GS, Gyawali CP: Functional dyspepsia: diagnostic and therapeutic approaches. Drugs 80 (2020): 1319-1336.
- Marsh A, Eslick EM, Eslick GD: Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. European journal of nutrition 55 (2016): 897-906.
- Staudacher HM, Nevin AN, Duff C, et al, Motility: Epigastric symptom response to low FODMAP dietary advice compared with standard dietetic advice in individuals with functional dyspepsia. 33 (2021): e14148.
- Altobelli E, Del Negro V, Angeletti PM, Latella G: Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients 9 (2017): 940.
- Pessarelli T, Sorge A, Elli L, Costantino A: The low-FODMAP diet and the gluten-free diet in the management of functional abdominal bloating and distension. Frontiers in nutrition 9 (2022): 1007716.
- Turco R, Salvatore S, Miele E, et al: Does a low FODMAPs diet reduce symptoms of functional abdominal pain disorders? A systematic review in adult and paediatric population, on behalf of Italian Society of Pediatrics. Italian journal of pediatrics 44 (2018): 1-14.
- Bellini M, Tonarelli S, Nagy AG, et al.: Low FODMAP diet: evidence, doubts, and hopes. Nutrients 12 (2020): 148.
- Manning LP, Yao C, Biesiekierski JR: Therapy of IBS: is a low FODMAP diet the answer? Frontiers in Psychiatry 11 (2020): 865.
- Bertin L, Zanconato M, Crepaldi M, et al.: The Role of the FODMAP Diet in IBS. Nutrients 16 (2024): 370.
- Biesiekierski JR, Tuck CJ: Low FODMAP diet beyond IBS: evidence for use in other conditions. Current Opinion in Pharmacology 64 (2022): 102208.
- Grammatikopoulou MG, Goulis DG, Gkiouras K, et al.: Low FODMAP diet for functional gastrointestinal symptoms in quiescent inflammatory bowel disease: a systematic review of randomized controlled trials. Nutrients 12 (2020): 3648.
- Duncanson K, Burns G, Pryor J, et al: Mechanisms of food-induced symptom induction and dietary management in functional dyspepsia. Nutrients 13 (2021): 1109.
- Jayasinghe M, Karunanayake V, Mohtashim A, et al.: The role of diet in the management of irritable bowel syndrome: a comprehensive review. Cureus 16 (2024).
- Maleesha J, Vinuri K, Ali M, et al.: The Role of Diet in the Management of Irritable Bowel Syndrome: A Comprehensive Review. Cureus 16 (2024).
- Minozzi S, Cinquini M, Gianola S, et al: The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. Journal of clinical epidemiology 126 (2020): 37-44.
- Zhang Y, Huang L, Wang D, et al: The ROBINS-I and the NOS had similar reliability but differed in applicability: a random sampling observational studies of systematic reviews/meta-analysis 14 (2021): 112-122.
- Lin L, Chu H: Quantifying publication bias in meta-analysis. Biometrics 74 (2018): 785-794.
- Potter MD, Duncanson K, Jones MP, et al: Wheat sensitivity and functional dyspepsia: a pilot, double-blind, randomized, placebo-controlled dietary crossover trial with novel challenge protocol 12 (2020): 1947.
- Boradyn KM, Przybylowicz KE, Jarocka-Cyrta EJAoN, Metabolism: Low FODMAP diet is not effective in children with functional abdominal pain: a randomized controlled trial 76 (2021): 334-344.
- Adibi P, Esmaillzadeh A, Daghaghzadeh H, et al: Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols diet is associated with increased risk of uninvestigated chronic dyspepsia and its symptoms in adults 69 (2021): 335-343.
- Buckle RL, Brown LC, Aziz I: P273 Randomised trial of traditional dietary advice in postprandial functional dyspepsia. BMJ Publishing Group; 2023. P273 Randomised trial of traditional dietary advice in postprandial functional dyspepsia | Gut
- Légeret C, Stienen Y, Furlano R, et al: Effectivity of treatment for children with functional dyspepsia 12 (2022): 1467.
- Cooke ZM, Resciniti SM, Wright BJ, et al.: Association between dietary factors, symptoms, and psychological factors in adults with dyspepsia: A cross-sectional study 35 (2023): e14684.
- Djatioetomo AK, Maharani AR, Djatioetomo YC, et al: Low-FODMAP diet on postprandial distress syndrome type of functional dyspepsia with mixed type of irritable bowel syndrome patient: A case report 4 (2024): e759.
- Buckle RL, Brown LC, Aziz IJN, Motility: Randomized trial in postprandial functional dyspepsia: Reassurance and diagnostic explanation with or without traditional dietary advice 36 (2024): e14733.
- Prabhakar D, Kini R, Premkumar KJIJoGI: Effectiveness of a low FODMAP diet and aerobic exercise in reducing epigastric symptoms among individuals with functional dyspepsia-A randomized controlled trial. 2025, 14: 57-63.
- Spiga F, Gibson M, Dawson S, et al.: Tools for assessing quality and risk of bias in Mendelian randomization studies: a systematic review 52 (2023): 227-249.
- Nejadghaderi SA, Balibegloo M, Rezaei N: The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Science Reports 7 (2024): e2165.
- Stang A: Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010, 25:603-605.
- Lin L, Chu H, Murad MH, et al.: Empirical comparison of publication bias tests in meta-analysis. Journal of general internal medicine 33 (2018): 1260-1267.
- Afonso J, Ramirez-Campillo R, Clemente FM, et al: The perils of misinterpreting and misusing “publication bias” in meta-analyses: an education review on funnel plot-based methods. Sports medicine 54 (2024): 257-269.
- Wang J, Yang P, Zhang L, et al: A low-FODMAP diet improves the global symptoms and bowel habits of adult IBS patients: a systematic review and meta-analysis. Frontiers in Nutrition 8 (2021): 683191.
- Tan VP: The low-FODMAP diet in the management of functional dyspepsia in East and Southeast Asia. Journal of gastroenterology hepatology 32 (2017): 46-52.
- McLaughlin J, Han G, Schalper KA, et al.: Quantitative assessment of the heterogeneity of PD-L1 expression in non–small-cell lung cancer. JAMA oncology 2 (2016): 46-54.
- Sedgwick P: Meta-analyses: what is heterogeneity? Bmj. (2015): 350.
- Wang X, Piantadosi S, Le-Rademacher J, et al: Statistical considerations for subgroup analyses. Journal of thoracic oncology 16 (2021): 375-380.
- Albuquerque AM, Santolia CB, Verma AJTJoCI: Concerns about the interpretation of subgroup analysis. (2022): 132.
- Rettura F, Lambiase C, Grosso A, et al.: Role of Low-FODMAP diet in functional dyspepsia:“Why”,“When”, and “to Whom”. Best Practice Research Clinical Gastroenterology 62 (2023): 101831.
- Pesce M, Cargiolli M, Cassarano S, et al.: Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World Journal of Gastroenterology: WJG 26 (2020): 456.
- Gibson PR, Halmos EP, So D, et al.: Diet as a therapeutic tool in chronic gastrointestinal disorders: lessons from the FODMAP journey 37 (2022): 644-652.
- Popa SL, Dumitrascu DI, Pop C, et al.: Exclusion diets in functional dyspepsia 14 (2022): 2057.
- van Lanen A-S, de Bree A, Greyling AJEjon: Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis 60 (2021): 3505-3522.
© 2016-2026, Copyrights Fortune Journals. All Rights Reserved