Tumor Lysis Syndrome and Cytokine Storm: Two Sides of The Same Coin?
Maria Saveria Rotundo1 and Roberto Verna*,1,2
1Research Center for Health and Social Wellness, Guglielmo Marconi University, Roma, Italy
2Academy for Health and Clinical Research, World Association of Societies of Pathology and Laboratory Medicine
*Corresponding author: Roberto Verna, Research Center for Health and Social Wellness, Guglielmo Marconi University, Roma, Italy.
Received: 03 October 2025; Accepted: 08 October 2025; Published: 18 October 2025
Article Information
Citation: Maria Saveria Rotundo and Roberto Verna. Tumor Lysis Syndrome and Cytokine Storm: Two Sides of The Same Coin?. Journal of Pharmacy and Pharmacology Research. 9 (2025): 122-134
Share at FacebookAbstract
Cytokines are small proteins released by cells, which can act synergistically or antagonistically to enable cellular interactions through pleiotropy (one cytokine exhibits multiple functionalities) and redundancy (multiple cytokines exert overlapping activities). They act in concert with specific cytokine inhibitors and soluble cytokine receptors to regulate immune response and their role in inflammatory processes is widely recognized. During tumor lysis, secondary to cancer cell death, the release of cytokines may occur massively, and an amplified tumor immunogenicity, which allows to elicit immune activation pathways and trigger a greater immune antitumoral response, is observed. The present search was performed to identify the molecular mechanisms involved in the cytokine release during cell death. The emerging data suggests the possibility of modelling the tumor microenvironment, converting an immunologically "cold" tumor into a "hot" tumor, thanks to novel combination strategies of immunotherapy and immunogenic cell death inducers, aimed to trigger a tumor-targeted adaptive response against immune silencing. However, significant effort is required to prevent and early diagnose tumor lysis syndrome and cytokine release syndrome due to the potential for life-threatening metabolic and immune-related adverse events.
Keywords
apoptosis, cell death, cytokine release syndrome, cytokine storm, immune checkpoint, tumor lysis syndrome
apoptosis articles, cell death articles, cytokine release syndrome articles, cytokine storm articles, immune checkpoint articles, tumor lysis syndrome articles
Article Details
Introduction
Tumor lysis syndrome (TLS) is a set of metabolic abnormalities resulting from tumor cell death with massive lysis and subsequent release of ions, nucleic acids, proteins, and metabolites into the bloodstream. It is considered a life-threatening oncological emergency, and it can occur during cytotoxic chemotherapy (within 12-72 hours), radiation therapy, cytolytic antibody therapy, and, finally, it can be also spontaneous in treatment-naïve patients. Laboratory tests may reveal a classical tetrad, consisting of hyperphosphatemia, hyperkalaemia, hyperuricemia and hypocalcaemia (with possible underlying nephropathy). However, other clinical manifestations, such as coagulopathy and disseminated intravascular coagulation, inflammation and tissue damages, have been reported [1]. Cytokine release syndrome (CRS) is a systemic inflammatory response triggered by the release of inflammatory cytokines from activated lymphocytes (T, B and natural killer NK cells) and/or myeloid cells (monocytes, macrophages, and dendritic cells DCs) or from non-immune cells, such as endothelial cells and tumor cells. It mainly derives from the immune system hyperactivation against infections or immunotherapy drugs. Initial symptoms are nonspecific (fever, nausea, fatigue, and muscle aches). "Cytokine storm" and CRS are often used interchangeably, although the latter more properly refers to the immunological phenomenon occurring during immunotherapy for haematological diseases. Despite the possible differences in triggers, clear similarities between the various cytokine storm syndromes suggest similar pathways in activating inflammation [2, 3].
Using a linear mathematical model, a small, but interesting, study described the details of a cytokine storm event, induced in six healthy volunteers with no previous history of immunodeficiency by administering TGN1412, a monoclonal antibody designed to stimulate regulatory T cells. Over the five-day measurement period, each of the nine analyzed pro- and anti-inflammatory cytokines induced or inhibited other cytokines. Although Tumor Necrosis Factor alpha (TNF-α) is a major pro-inflammatory factor, Interferon gamma (IFN-γ) and other cytokines (interleukins IL-2, IL-4, IL-8, and IL-12) exhibited the most rapid response to TGN1412, peaking approximately six hours after TGN infusion. In this time T cell, monocyte, and platelet concentrations plummeted. A sudden burst of cytokine production by activated cells could lead to their apoptotic death and reduction in cell population. The neutrophil profile resembled the slower IL-6 response, peaking 1–2 days after infusion, and neutrophil concentrations remained above their reference range for over 10 days. C-reactive protein concentrations, a strong marker of inflammation, peaked 2–3 days after infusion and returned to normal levels approximately 10 days after the event. Cells of the innate and adaptive immune systems were involved in all phases of the TGN1412-induced cytokine storm [4]. The cytokine storm model has been shown, in other studies, to be subject to factors such as the microbiome, genetic characteristics, and underlying pathologies [2, 3]. Cytokine release may occur during tumor lysis and induce a greater immune antitumoral response. Some types of tumor cell death are more involved than others in triggering a cytokine storm and are capable of amplifying tumor immunogenicity. Based on the above rationale, this review aimed to investigate the molecular processes shared by TLS and CRS, outlining the current state of clinical research and future challenges.
Methods and Materials
Eligibility criteria for Systematic Search
The topic of the included studies was the identification of the molecular mechanisms involved in the cytokine release by in vitro experiments on cell lines and animal models, focusing on cell death pathways and modulation of immune responses during anticancer therapies and consequent tumor lysis. Secondary research was performed to synthesize existing clinical practice guidelines to consolidate knowledge on TLS and CRS.Abstracts with insufficient information detailing study design, experiments’ characteristics and outcomes were not considered. Articles written in languages other than English were excluded.
Search Strategy, Data Extraction and quality evaluation
The present search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA 2020 checklist and their extension) [5]. No study involving human participants and requiring ethics committee approval, based on the Declaration of Helsinki and its subsequent revisions, was conducted during the present investigation. The public databases MEDLINE (URL: https://www.nlm.nih.gov/medline/index.html), PubMed (URL: http://www.ncbi.nlm.nih.gov/pubmed) and Embase (URL: www.embase.com) were searched for entries from January 01, 1990 until August 31, 2025. A Google academic search (URL: scholar.google.com) was also performed to track relevant references. The search included the following keywords: (Tumor lysis or Cytokine release or Cytokine storm) AND (chemotherapy or immunotherapy or radiotherapy) AND cell death AND signal transduction pathway. The studies were examined independently by two investigators (MSR, RV) to verify concordance with the eligibility criteria.
Results
Study selection
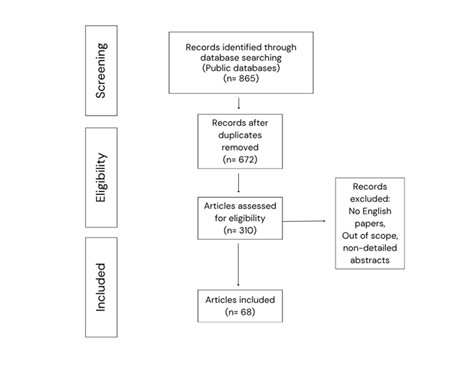
Published within the 1996-2025 timeframe, 865 articles were included in the bibliographic databases. Possible duplicates were excluded. The remaining 672 articles were further reviewed, as potential candidates for the present systematic search, up to the final inclusion of 68 articles. The searching and selection process is outlined in Figure 1.
Epidemiology
TLS is more common in patients with non-Hodgkin lymphoma and other hematological malignancies, particularly Burkitt lymphoma, acute lymphoblastic leukemia, and acute myeloid leukemia. Among patients developing TLS, only a small percentage will experience clinically significant symptoms, with consequent higher mortality, according to the studies on onco-hematological diseases [6, 7]. High proliferative index (a measure of the number of cells in a tumor that are dividing), high tumor burden (referred to the number of cancer cells, the size of a tumor, or the amount of cancer in the body) and high sensitivity to cancer treatments and radiation therapy are considered the major risk factors. Moreover, bone marrow infiltration, intensity of the initial antitumor therapy, dehydration, reduced blood volume, hypotension, renal infiltration, urinary outflow obstruction, pre-existing nephropathy, acidic urine, and exposition to nephrotoxins may contribute to triggering the syndrome [8].
In addition to autoimmune diseases/genetic syndromes and infections (most notably coronavirus disease COVID-19), Cytokine storm has also been observed secondary to tumors and antitumoral treatment, especially during immunotherapy with checkpoint inhibitors and chimeric antigen receptor T-cell (CAR T) therapies. The incidence of CRS is not well defined due to the multiple unspecific symptoms, resulting in difficult differential diagnosis. Overall, CRS has been observed in 50–90% of cases in cancer immunotherapy studies involving T cells, although a correlation between its occurrence and severity did not predict clinical response. Type of therapy, dose administered, underlying disease (disease burden can predict the severity of CRS in patients undergoing T-cell immunotherapy), patient characteristics (higher incidence in children than adults), strength of T-cell activation, degree of T-cell expansion can influence the onset of CRS. A "first-dose effect” without recurrence after subsequent administrations could be due to an initial higher disease burden [9, 10].
Classification
The National Cancer Institute's Common Toxicity Criteria (URL: https://dctd.cancer.gov/research/ctep-trials/for-sites/adverse-events) version 2.0 assigns TLS a grade of 0 (absent) and a grade of 3 (present), while the Common Terminology Criteria for Adverse Events 3.0 assigns a grade of 3 if present and a grade of 5 if death occurs, and version 5.0 includes a grade of 4 (life-threatening consequences; urgent intervention indicated). Classifications for TLS were also discussed by Hande-Garrow, Cairo-Bishop and by an expert panel in paediatric and adult hematologic malignancies and showed in Table 1 [11-13]. CRS can occur with fever, tachypnea, headache, tachycardia, hypotension, rash, hypoxia and other disorders, including cardiovascular, pulmonary, renal and neurological manifestations. CRS may also overlap with macrophage activation syndrome/hemophagocytic lymphohistiocytosis. Circulating cytokine levels may be useful for diagnosis, but a multiple cytokine panel test is expensive. The acute phase reactant C-reactive protein is a reliable surrogate for IL-6 bioactivity, and it allows to identify patients at risk for severe CRS [14].
Table 1: Diagnostic criteria for TLS
|
Hande-Garrow |
Cairo-Bishop |
Consensus Panel |
|
|
Laboratory criteria |
Two of the following changes: |
Two of the following changes: |
|
|
• a 25% increase in serum P, K, UA, or BUN concentrations above the upper limit of normal, or |
• a 25% increase in serum P, K, UA, or BUN concentrations above baseline, or |
• as per Cairo-Bishop, but hypocalcemia was ruled out. |
|
|
• a 25% decrease in serum Ca below the lower limit of normal |
• a 25% decrease in serum Ca below baseline |
• renal failure was added as a risk factor for the development of TLS. |
|
|
within 4 days of chemotherapy |
within 3 days before or 7 days after starting chemotherapy |
||
|
Clinical criteria |
L-TLS + one or more of the following: |
L-TLS + one or more of the following: |
|
|
• K>6 mmol/L |
• renal impairment with serum Cr ≥1.5× upper normal limit |
||
|
• Cr>221 μmol/L |
• cardiac arrhythmias/sudden death |
||
|
• Ca <1.5 mmol/L |
• seizures |
||
|
• life-threatening arrhythmia or sudden death |
Abbreviations:
BUN: blood urea nitrogen; Ca: calcium; Cr: Creatinine; K: potassium; L-TLS: laboratory tumor lysis syndrome; TLS: tumor lysis syndrome; P: phosphorus; UA: uric acid.
Diagnostic criteria for CRS and severity are showed in Table 2.
Table 2: Diagnostic criteria for CRS
|
National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v5.0) |
|||||
|
GRADE |
1 |
2 |
3 |
4 |
5 |
|
Fever +/- constitutional symptoms |
Hypotension responsive to fluids; hypoxia responsive to <40% O2 |
Hypotension requiring 1 vasopressor; hypoxia requiring >40% O2 |
Life-threatening consequences: urgent intervention indicated |
Death |
|
|
Daniel W. Lee and colleagues |
|||||
|
GRADE |
1 |
2 |
3 |
4 |
5 |
|
Symptoms are not life-threatening and require only symptomatic treatment (e.g., fever, nausea, fatigue, headache, myalgia, malaise) |
Symptoms require and respond to moderate intervention (oxygen requirement <40% or hypotension responsive to fluids or low-dose vasopressors, or grade 2 organ toxicity) |
Symptoms require and respond to aggressive intervention (oxygen requirement ≥40% or hypotension requiring high-dose or multiple vasopressors, or grade 3 organ toxicity or grade 4 transaminitis) |
Life-threatening symptoms/need for ventilatory support/grade 4 organ toxicity (excluding transaminitis) |
Death |
|
Treatment and Prevention
Treatment and/or prevention of TLS include: intravenous hydration to maintain diuresis (70-100 ml/m2 per hour) and improve kidney function; diuretics (use limited to some cases); allopurinol, rasburicase, febuxostat for hyperuricaemia; correction of electrolyte imbalance (such as sodium polystyrene sulfonate and/or glucose with insulin for hyperkalaemia; aluminum hydroxide, calcium carbonate, sevelamer hydroxide for hyperphosphatemia; calcium gluconate for hypocalcemia) [15]. Intensive Care Unit admission should be considered for all patients with CRS. Low-grade CRS is treated symptomatically with antihistamines, antipyretics, and fluids. Other therapies are vasopressors, oxygen and empiric antibiotics. Severe CRS is a life-threatening condition. Immunosuppression should be used in all patients with Grade 3 or 4 CRS. Tocilizumab, a humanized monoclonal antibody (Immunoglobulin G1κ) targeting human Interleukin-6 receptor (IL-6R), prevents IL-6 binding to both cell-associated and soluble IL-6Rs and thus inhibits both classical and trans IL-6 signaling. The drug is administered intravenously at a dose of 8 mg/kg for adults and 12 mg/kg for patients weighing <30 kg, aged 2 years and older, up to a maximum of 800 mg per dose, with an interval between consecutive doses of at least 8 hours. A second dose of tocilizumab and/or a second immunosuppressive agent, such as corticosteroids, should be considered if the patient does not improve or stabilize within 24 hours of tocilizumab infusion. Corticosteroids (methylprednisolone at initial dose of 2 mg/kg/day, dexamethasone at a dose of 0.5 mg/kg - maximum 10 mg/dose) are the second-line therapy due to their possible detrimental effect on immunotherapy activity and the faster response achieved with tocilizumab. However, they are considered useful against neurological symptoms for the improved blood-brain barrier penetration. Other immunosuppressive agents are anti-TNF-α monoclonal antibodies (infliximab) and soluble inhibitors of the TNF receptor TNFR (etanercept) and IL-1R (anakinra) [14].
Pathophysiology
In TLS, rapid lysis resulting from cell death leads to the release of massive amounts of intracellular contents into the bloodstream, including anions, cations, proteins, and nucleic acids. The pathophysiology of CRS is still unclear. Among the immune system cells, able to recognize pathogens and respond to microbial invaders, neutrophils, macrophages, and NK cells are the most frequently implicated in the pathogenesis of the cytokine storm. IFN-γ, IL-1, IL-6, TNF, and IL-18 are key cytokines [16, 17]. The membrane-bound IL-6R does not have intracellular signaling domains and the downstream signal transduction is triggered by interaction between the IL-6-IL-6R complex and the membrane-bound glycoprotein 130 (gp130), provided with intracellular domain (cis-signaling). Gp130 is ubiquitously expressed, while the membrane-bound IL-6R expression is restricted to the immune cells. JAKs (Janus kinases) and STAT3 (signal transducer and activator of transcription 3) as well as Akt-mTOR (mammalian target of rapamycin) and MAPK-ERK (mitogen-activated protein kinase–extracellular signal-regulated kinase) pathways are involved. When circulating level of IL-6 raises, the cytokine binds to the soluble form of IL-6R and the subsequent interaction with gp130 potentially occurs on all cell surfaces (trans-signaling), inducing the JAK-STAT3 cascade also in cells not expressing membrane-bound IL-6R, such as endothelial cells. This event opens the way to systemic hyperinflammation with secretion of MCP-1 (monocyte chemoattractant protein 1), IL-8, and additional IL-6, increased vascular endothelial growth factor (VEGF) and reduced E-cadherin expression on endothelial cells, with vascular hyperpermeability [18].
In response to tissue injury or pathogenic stimuli, TNF-α, expressed by macrophages, T lymphocytes, mast cells, granulocytes, NK cells, fibroblasts, neurons, keratinocytes, and smooth muscle cells, can bind to two receptor types, TNFR1 and TNFR2. The complex I, assembled at TNFR cytoplasmic domains, comprises TRADD (TNFR1-associated death domain), TRAF2 (TNF receptor-associated factor-2), RIPK1 (receptor-interacting serine/threonine protein kinase 1), E3 ubiquitin ligases BIRC2, BIRC3 (cIAP1/2, cellular inhibitor of apoptosis protein) and the linear ubiquitin chain assembly complex LUBAC. It recruits and activates the transforming growth factor (TGF)-β-activated kinase 1 TAK1 complex, and the inhibitor of κB: IκB kinase (IKKβ) complex. The induction of signalling complex I leads to expression of AP1 (activation of downstream JUN N-terminal kinase JNK) and p38, as well as AP1 transcription factors via mitogen-activated kinases (MAPK), triggered by TAK1, and canonical nuclear factor κB (NF-κB, activated by IKKβ) target genes, involved in inflammation, host defence, and cell proliferation and survival. In contrast to complex I, complexes IIa and IIb lead to activation of a caspase (proteolytic enzymes belonging to the cysteine-aspartate protease family that cleave target proteins after an aspartic acid residue, resulting in protein and DNA fragmentation) cascade that results in TNF-induced cell death via apoptosis, while complex IIc activates the necroptosis effector mixed lineage kinase domain-like protein (MLKL) by a RIPK3-dependent mechanism. When the survival pathway is inhibited, TRADD:TRAF2:RIPK1 detaches from the membrane-bound TNFR1 signaling complex and recruits the Fas-associated death domain-containing protein (FADD) and procaspase-8 (also known as complex II). Procaspases-8 interact via their tandem death-effector domains (DEDs), thus promoting the initiation of apoptotic cell death [19].
IL-18 and IL-1β are activated from their precursors by inflammasomes, multimolecular cytosolic sensors for pathogenic microorganisms and stressors, with consequent induction of pyroptosis mediated by caspase-1. IL-18 (produced by macrophages and DCs), IL-12 and IL-15 stimulate the secretion of IFN-γ by T lymphocytes and NK cells, thus promoting Th1 (T helper 1) -type inflammatory responses. IL-1β and IL-18 are also potent inducers of IL-6 secretion from macrophages. Chemokines are a class of cytokines that contribute to a variety of immune cell functions, including leukocyte recruitment and trafficking. IL-10 and natural cytokine antagonists, such as IL-1R antagonist, can limit systemic off-target effects of a dysregulated trafficking involved in hyperinflammation. IL-10 inhibits the production of TNF, IL-1, IL-6, and IL-12 and reduces antigen presentation. Furthermore, in mice lacking IL-10, infection can induce a cytokine storm. Plasma proteins, such as complement proteins and other inflammatory mediators, may also contribute to the pathogenesis of cytokine storm [20].
Cell Death
Traditional classifications of cell death distinguish: Programmed Cell Death (PCD) - among which the main one is Apoptosis -, Autophagy and Necrosis. Apoptosis, or type 1 cell death, is an active, energy-dependent, genetically regulated PCD mechanism accompanied by characteristic changes in cell morphology, especially in the nucleus. Apoptosis is triggered by two different pathways, intrinsic and extrinsic, both conveying in the execution pathway. In response to various extra and intracellular stress, cytochrome c is released from the mitochondrial intermembrane space into the cytosol (mediated by Bcl-2-Associated X protein (Bax)/ BCL2 associated agonist of cell death (Bak) insertion into mitochondrial membrane). The anti-apoptotic proteins Bcl-2 and Bcl-xL (Bcl-2 family member) block the release of cytochrome c. Apoptosome, composed by cytochrome c, Apaf–1 and procaspase-9, triggers caspase 9 with subsequent activation of caspase-3 signaling caspase cascade. In the extrinsic apoptosis, perturbations in the extracellular microenvironment induce the binding of the extracellular ligand TNF-α and Fas ligand (FasL) to their respective receptors, TNF and Fas (called death receptors), with the subsequent apoptotic protein complex formation. Caspase-8 and caspase-9, activated by intrinsic and extrinsic pathways respectively, cleave and activate caspase-3 during the execution pathway (effector caspases, caspase-3, -6, and -7), leading to the degradation of intracellular components (final stage) by activated proteases and endonucleases. Physiologically, autophagy has catabolic roles, helping maintain cellular homeostasis and recycle nutrients while removing toxic cellular components, with the formation of a double isolation membrane, or phagophore, a structure that sequesters cytoplasmic components for degradation. Under stress conditions, its dysregulation can cause cell death, called autophagic cell death, or type 2 cell death, mediated by mTOR inhibition. The phagophore, containing the cytoplasmic material (autophagosome), expands until it fuses with lysosomes to form autolysosomes, where the sequestered contents are degraded by lysosomal enzymes. Necrosis is often caused by external stimuli that induce cell membrane disruption and inflammation. Under stressful conditions, cells die through an unregulated necrotic process. However, necrosis can also include signs of controlled processes, such as mitochondrial dysfunction, increased generation of reactive oxygen species, adenosine triphosphate (ATP) depletion, proteolysis by calpains and cathepsins, and premature rupture of the plasma membrane. Cyclophilin D (CypD), or peptidyl prolyl isomerase F (PPIF), is a mitochondrial matrix protein, component of the "permeable pore transition complex" (PTPC, a complex assembled at the junction of the inner and outer mitochondrial membranes). The opening of nonspecific channels that causes the dissipation of the internal mitochondrial transmembrane potential, with sudden loss of impermeability to small solutes, osmotic dysfunction, followed by impaired ATP production and the release of pro-necrotic factors into the cytoplasm, so called "mitochondrial permeability transition" (MPT), is mediated by Bax or Bak and not by CypD in apoptosis, while it is CypD-dependent in necrosis. Lysosomal proteases (calpains and cathepsins), apoptosis-inducing factor (AIF), lysosomotropic toxins and radical oxygen species (ROS) appear to play an important role in death processes, also by inducing lysosomal membrane permeabilization (LMP) [21].
Immunogenic cell death (ICD), Pyroptosis and Necroptosis are subtypes of PCD frequently recognized responsible for triggering CRS during tumor lysis. In ICD, an inflammatory type of PCD, tumor cells undergo apoptosis while converting them from non-immunogenic to immunogenic, activating host-specific anti-tumor immunity. Damage-associated molecular patterns (DAMPs), among which ATP, high-mobility group box 1 (HMBG1), sessile endoplasmic reticulum proteins and heat shock proteins (HSP90 and HSP70) are released from dying cells and attract immune cells to the site of cell death, promoting antigen presentation and immune activation. Furthermore, IFN-γ and TNF-α released by effector T cells attract and activate other immune cells, including natural killer cells and macrophages, which detect and eradicate tumor cells. Calreticulin, one of the DAMP molecules which is normally in the lumen of the endoplasmic reticulum, is translocated on the surface of dying tumor cells and promotes their phagocytosis by DCs and other phagocytic cells. Pyroptosis is a lytic form of PCD, regulated by caspases, particularly caspase-1 and caspase-11 (in mice) and caspase-1/4/5 (in humans). In response to pathogen-associated molecular patterns (PAMPs) or DAMPs as cellular stress signals, pattern recognition receptors (PRRs) on the membrane of antigen-presenting cells (APCs) are activated and induce the translocation of NF-κB to the cell nucleus to initiate transcription of caspase-1, NLR family pyrin domain containing 3 (NLRP3), pro-IL-1β, and pro-IL-18. A cytosolic protein complex consisting of PRRs, adaptor proteins, and caspase-1 is assembled (inflammasome), and the activated caspase-1 cleaves gasdermin D (GSDMD), which forms membrane pores. Caspase-1 cleaves also the proforms of proinflammatory cytokines, such as IL-1β and IL-18, into their active forms, which are released through the GSDMD pore for recruitment of immune cells to the site of infection or injury. IL-1β mediates the recruitment of neutrophils and T lymphocytes and secondary cytokines, such as IL-6 and TNF, are released from epithelial and endothelial cells. Elevated levels of IL-18 stimulate the production of IFN-γ by T lymphocytes and NK cells. Furthermore, the binding of IL-1β to the IL-1R1 and of IL-18 to the IL-18R activates the NF-κB signaling pathway, creating a positive feedback loop that amplifies the inflammatory response. Necroptosis is a lytic form of PCD, which occurs through a signaling cascade involving the activation of the RIP receptor-interacting protein family, when apoptosis mediated by caspase-8 is suppressed. The protein kinases RIPK1 and RIPK3 are activated by the binding of TNF-α to TNFR1, leading to mitochondrial instability. RIPK1 recruits RIPK3 through the RIP homotypic interaction motif (RHIM) to form complexes, called necrosomes, which promote the oligomerization and phosphorylation of MLKL. After phosphorylation, MLKL assembles into oligomers and is translocated from the cytosol to the plasma membrane, leading to formation of membrane pores and subsequent membrane disruption [22-24].
Cell Death Induced by Antitumor Therapy
An important modality of action of many chemotherapies is the induction of DNA damage. When cellular repair mechanisms (base excision repair, nucleotide excision repair, mismatch repair, non-homologous end-joining and homologous recombination) are impaired, the DNA damage, caused by chemotherapy, leads to the activation of cell death pathways. Instead, an increased expression of repair genes correlates with cell survival, and it can produce chemotherapy resistance. Chemosensitivity can differ in apparently identical tumor cells also due to a different balance between pro- and anti-apoptotic proteins: tumor cells defined as "primed" for apoptosis are more chemosensitive than "unprimed" cells [25-30]. Among the direct effects of ionizing radiation, the accumulation of chromosomal aberrations and defective repair can trigger the so-called mitotic catastrophe (proliferative death), while in the interphase death, cells cease to divide after exposure to radiation and begin to die within a few hours by disruption of membrane structure and components of cellular energy metabolism. Furthermore, in both cellular and animal experiments, following radiation exposure a greater propensity for apoptosis and/or autophagy or tumor cell senescence derived from the activation of p53-dependent metabolic pathways [31-33].
By adding immune checkpoint inhibitors (ICI), such as anti-cytotoxic T lymphocyte-associated protein 4 (CTLA4) or anti- Programmed Cell Death Protein 1 (PD-1), to local radiotherapy, ICD can occur not only in tumor cells in the radiation area, but also in the other tumor cells outside the area, thanks to the modulation of the host immune system (immune-mediated tumor rejection) [34, 35]. The epidermal growth factor receptor (EGFR) signaling appears involved in the malignant behaviour of tumor cells and in modifying the tumor microenvironment to promote cancer progression. Activation of EGFR tyrosine kinase, through binding of EGF to its receptors, leads to stimulation of the PI3K-AKT-mTOR and RAS-RAF-MEK-ERK axes, involved in cell growth and proliferation. In EGFR-dependent tumors, the PI3K-AKT-mTOR inhibition reduces Mcl-1 (a pro-survival member of the Bcl-2, B-cell lymphoma 2, family), while the MEK inhibition increases the pro-apoptotic protein BIM (B-cell Lymphoma 2-like protein 11), inducing apoptosis. In a mouse model the EGFR blockade by administration of an antibody specific for the extracellular domain of murine EGFR, called 7A7, induced a greater proapoptotic effect on tumor metastases compared to an EGFR tyrosine kinase inhibitor, called AG1478. Moreover, it was also able to induce apoptosis with immunogenic potential in an Fc (fragment crystallizable region) -independent manner, mobilizing the T cells to metastatic sites and stimulating potent proapoptotic cytotoxic T lymphocyte (CTL) activity. Therefore, some antibodies have proven ability to induce specific antitumor immunity, not limited to the target antigen, but against a variety of unknown tumor-derived antigens through activation of adaptive immunity. Consistent with “in vivo” immunogenic apoptosis experiments, EGFR inhibition by 7A7 is sufficient to induce the translocation of immunogenic molecules to the cell surface. 7A7 induced early phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α), a key step in anthracycline-related immunogenic apoptosis, and increased the expression of HSP70 and HSP90 in the plasma membrane. Administration of 7A7 enabled DC maturation subsequent to antigen release from dying tumor cells and significantly increased CD8+ T cell (secreting IFN-γ and granzyme) infiltration into lung metastases.
It has also been hypothesized that the Fc region of cetuximab, an antibody directed against EGFR, may contribute to adaptive immune activation through the interaction between NK cells and DCs. EGFR inhibition promoted TNF-α activation in glioblastoma and increased TNF-mediated lung epithelial cell apoptosis and lung damage. Erlotinib, an EGFR inhibitor, however, reduced lung inflammation in an inflammatory marker model of advanced lung cancer by suppressing RIPK3-dependent necrosis. In one study, phosphorylation of the death domain of TNFR1, performed by EGFR tyrosine kinase activated by EGF binding to its receptor, suppressed the subsequent interaction between TNFR1 and RIPK1, resulting in reduced polyubiquitination of RIPK1, which in turn downregulates the TNF-α signaling pathway [36-40]. In CAR-T therapy (effective in treating blood cancers, such as lymphoma and leukaemia, and promising in autoimmune diseases, such as systemic lupus erythematosus), T cells isolated from the patient's blood via leukapheresis are engineered to express the CAR, expanded, and reinfused to the patient. CAR-expressing T cells bind to a specific antigen on tumor cells independently of the major histocompatibility complex (MHC). Before CAR-T cell administration, lymphodepleting chemotherapy (typically fludarabine and cyclophosphamide) is administered. Forty-eight hours later, the CAR-T cells are thawed and immediately infused intravenously to the patient. Cytokines released by interactions between tumor cells and immune cells can mediate various cytotoxic pathways. IFN-γ signaling in tumor cells can promote antigen presentation and MHC class I for T-cell-mediated killing and contribute indirectly to cytotoxicity through upregulation of the cytokine TNF-α. Other members of the TNF superfamily can mediate cell death, such as TNF-related apoptosis-inducing ligand (TRAIL) and FasL.
After conjugation with a tumor cell, T lymphocytes release serine proteases called granzymes and perforin, which form transient pores in the tumor cell membrane in a calcium-dependent modality, allowing the influx of granzymes into the cytosol, capable of cleaving and activating apoptosis-inducing proteins. Among the five granzymes identified in humans, the most studied granzyme B (GZMB) could be required for T-cell-mediated apoptosis in tumor cells. SERPINB9 (Serine Proteinase Inhibitor, Clade B, Member 9), an inhibitor of both GZMB and protease 9, would play a role in tumor resistance [41]. In bispecific T-cell extender (BiTE) fusion proteins, whose prototype is Blinatumomab (Blincyto®), single-chain variable fragments (scFv) from two antibodies are linked by a peptide linker: one fragment binds to the tumor-associated antigen and the other fragment binds to the CD3 subunit of the T-cell receptor (TCR) complex. BiTE acts as a bridge, guiding the T cells into proximity with tumor cells to promote MHC-independent T cell-mediated cytotoxicity and the release of cytokines: IFN-γ, TNF-α, IL-6, and IL-10. The CD3+ cells, particularly CD3+CD4+ helper T cells, CD3+CD8+ cytotoxic T cells, and CD3+ NKT cells, can be activated to become cytotoxic effector cells. Co-stimulation of T cells by IL-2 or anti-CD28 antibodies is not required. Furthermore, the concomitant binding of BiTE to tumor-associated antigen and CD3 in the TCR complex would form a cytolytic synapse between T cells and tumor cells, with release of perforin and granzyme, as well as possible caspase activation [42, 43].
Immune checkpoints are cell surface proteins, which maintain the homeostatic balance between suppression and upregulation of the immune system. PD-1 (CD279) is an inhibitory receptor expressed on the surface of activated T lymphocytes, B lymphocytes, NKT cells, NK cells, monocytes, myeloid-derived suppressor cells, tumor-associated macrophages, and DCs. Two ligands for PD-1 are: programmed death ligand 1 or PD-L1 (B7-H1) and programmed death ligand 2 or PD-L2 (B7-DC). PD-L1 is expressed on T lymphocytes, B lymphocytes, APC, and in some non-lymphoid tissues and normal and malignant tumor cells. The interaction between PD-1 and PD-L1 reduces the production of IL-2 and IFN-γ and blocks T cell proliferation and survival through a process mediated by the SH2-containing phosphatase 2 (SHP2) and the PI3Kinase/Akt downstream signaling. Furthermore, the interaction reduces anti-apoptotic factors and upregulates pro-apoptotic factors, leading to T cell apoptosis, and induces downregulation of the TCR through the E3 ubiquitin ligase Cbl-b expression. The PD-1/PD-L1 axis has a role in the expansion and maintenance of immunosuppressive regulatory T cells (Treg) and in the conversion of CD4+ T cells to Treg. The PD-1/PD-L1 interaction mediates resistance to tumor cell killing by CD8+ T cells. As anti-apoptotic receptor on tumor cells, PD-L1 prevents apoptosis induced by Fas ligation and it protects tumor cells from IFN-γ-mediated cytotoxicity through STAT-3/Caspase-7-dependent signaling.
ICIs enhance immune activity against tumor cells and, aberrantly, against non-tumor host cells, resulting in inflammatory side effects, also known as immune-related adverse events (irAEs). The CTLA-4 competitively inhibits the binding of CD28 to CD80/CD86, attenuating T cell activation, when the antigen-specific T cell receptor interacts with an MHC-copresented peptide. Conversely, CTLA-4 blockade increases T cell activation and proliferation, including for tumor-infiltrating effector T cells, and reduces Treg function. The monoclonal antibody Ipilimumab binds to CTLA-4 and prevents its interaction with CD80/CD86. A greater efficacy emerged by the combination of ipilimumab and nivolumab thanks to the increased expansion of CD8 effector T cells. The inhibitor of lymphocyte activation gene 3 (LAG-3), expressed on Tumor-infiltrating lymphocytes (TILs, activated CD4+ and CD8+ T lymphocytes), Treg cells, NK cells, γδT cells, NKT cells, and DCs, binds to the MHC-II receptor with greater affinity than the CD4 T cell receptor, blocking TCR signaling and cytokine secretion and leading to immune suppression. LAG-3 is expressed at lower levels on naïve CD8+ T cells and it increases on activated CD8+ T cells. LAG-3 promotes Treg cell differentiation. Combination therapy with ICIs is also associated with CRS [44, 45].
The Tumor Microenvironment (TME): A Battlefield Where Tumor Lysis Can Trigger a Cytokine Storm
According to the concept introduced by Ioannides in 1993, Tumor microenvironment (TME) refers to the local environment of tumor initiation and progression and comprises tumor cells, stromal cells, immune cells (T lymphocytes, macrophages, NK cells, bone marrow-derived suppressor cells, mast cells, neutrophils, B lymphocytes, DCs, tertiary lymphoid structures resulting from lymphoid neo-organogenesis) and their cytokines, and the extracellular matrix. TILs, isolated from tumor tissue, are categorized into two functional groups: positive regulatory immune cells, such as DCs, CD8+ T cells, and NK cells, and negative regulatory immune cells, such as tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), Treg cells, myeloid-derived suppressor cells, and tumor-associated neutrophils (TANs). The third type of lymphoid tissue, called tertiary lymphoid tissue and distinct from primary lymphoid tissue (thymus and bone marrow) and secondary lymphoid tissue (spleen, lymph nodes, tonsils, etc.), consists of multiple immune cells, including T and B cells, DCs, and lymphatic vessels, which promote immune cell infiltration into solid tumors [46].
To induce immunological tolerance, tumor cells release extracellular signals influencing the TME. VEGF inhibits T-cell infiltration by suppressing intracellular adhesion molecule-1 (ICAM-1), with consequent tumor growth stimulation. Moreover, it inhibits DC maturation and activates Treg cells and myeloid suppressor cells. Tumor vasculature and dense fibrogenic extracellular matrix can limit CAR T cell penetration into solid tumors. The VEGF inhibitor bevacizumab in combination with CAR T cells improved tumor infiltration and antitumor efficacy in a preclinical model of human neuroblastoma [47]. Immunosuppressive immune checkpoint pathways, involving PD-1/PD-L1 and CTLA-4, as well as immunosuppressive factors, such as IL-10 and TGF-β, are responsible for features of the so-called "cold tumors". The expression of PD-L1 on tumor cell surface contributes to immune cell inhibition (intrinsic immunosuppression). T-cell exhaustion is sustained by the binding of Gal9 and PD-L1 to T-cell immunoglobulin-mucin domain 3 (TIM-3), and PD-1, respectively. Although immunotherapy with ICIs blocks the PD-1/PD-L1 or CTLA-4 pathway, thereby "normalizing" the immune system, a large percentage of patients remains refractory. A poor infiltration of the TME by CTLs, which often correlates with a low tumor mutational burden, and therefore a low neoantigen burden, and limited expression of PD-L1, can determine ICI resistance in cancer patients [48]. In "hot" tumors deficiencies in Mismatch Repair (MMR) genes MLH1, MSH2, MSH6, and PMS2, resulting in high microsatellite instability (i.e., short repeat sequences: repetitive sequences of fewer than 10 nucleotides in the DNA genome), and high tumor mutational burden (the number of nonsynonymous mutations occurring in the tumor genome, reflecting the degree of genomic variation in the tumor) allow to acquire multiple de novo antigens, which can boost the tumor immunogenicity. In these tumors, immune activation pathways, such as CD28/B7, CD40/CD40L, with an increased number of immune cells, mainly T lymphocytes, NK cells and DCs, fewer immunosuppressive factors and a strong (adaptive) immune response stimulated by immune activation factors, are involved.
The abundant T-cell infiltration of "hot" tumors is a promising target for immunotherapy, aiming to enhance the antitumor surveillance and a key challenge for researchers is transforming "cold" tumors into "hot" ones. Combination therapy of CAR T cells with checkpoint inhibitors and the use of redirected universal cytokine-killing TRUCK T cells (fourth-generation CAR T cells) to contrast the immunosuppressive microenvironment through local cytokine secretion are new strategies of TME modulation for enhancing the immune system's ability to recognize and fight against tumor. TRUCK cells are engineered to target tumor cells using a CAR and simultaneously release cytokines, such as IL-12, IL-15, or IL-18, which activate and recruit other immune cells to the tumor site, creating a local inflammatory environment. They prolong the persistence and amplification of TILs and NK cells, improving their killing capacity and overall antitumor efficacy. Several standard-of-care (SOC) therapies, which include conventional chemotherapy, radiotherapy, and/or targeted anticancer drugs, are considered ICD inducers, due to their proved ability to trigger a tumor-targeted adaptive immune response, and they resulted capable of converting an immunologically "cold" tumor (insensitive to ICIs) into a "hot" tumor, with an abundant CTL infiltrate and therefore responsiveness to ICIs. Among physical inducers of ICDs, radiotherapy was administered in the context of CTLA4 or PD-1 blockade, along with cyclophosphamide or doxorubicin, reaching increased anticancer therapeutic benefits [49-54]. DAMP excretion occurs during necrosis in inflammatory and/or pathological conditions and during infections, while PAMPs are produced by viral or bacterial components. More recently, DAMP production has been observed also in tumor cells undergoing ICD after chemotherapy or radiotherapy.
The DNA-binding protein HMGB1 (High Mobility Group Box 1), or histone chromatin-nonbinding nuclear protein (HCNP), deriving from the nucleus and excreted by cells in advanced stages of death, triggers inflammation by stimulating the production of proinflammatory cytokines from APCs through its binding to several surface receptors, including the receptor for advanced glycation end products (RAGE), the Toll-like receptors TLR2, TLR4, TLR9, and TIM3. HMGB1 is an intrinsic sensor of oxidative stress, and its immunomodulatory properties may be determined by its redox state. HMGB1 binding to TLR4 on APCs contributes to suppress tumor development, while secreted HMGB1 may induce inflammation that facilitates tumor progression. Toll-like receptors (TLRs) are primitive PRRs found on various immune and tissue cells. They detect PAMPs or DAMPs and can trigger cytokine storms via two key pathways: the MyD88-MAPK canonical pathway (producing pro-inflammatory cytokines like TNF, IL-1β, and IL-6) and the non-canonical TRIF-IRF3 pathway (inducing type I interferons: IFN-α and β). TLRs also regulate NF-κB to further promote cytokine production. Additionally, cytokines activate the TCR/BCR/NF-κB pathway, amplifying the inflammatory response. The NLRP3 inflammasome pathway requires two signals: the first signal leads to the activation of NF-κB and subsequent upregulation of NLRP3, pro-IL-1β, pro-IL-18 and caspase-1; the activation signal (signal 2) involves the NLRP3 inflammasome, with recruitment of ASC, maturation of IL-1β, secretion of IL-18 and triggering of GSDMD-mediated pyroptosis.
ATP, recognized as a "find-me" signal by purinergic P2Y2 receptors on phagocytes, enables receptors’ migration to inflamed sites. ATP, released by tumor cells treated with chemotherapeutic agents, is essential for effective antitumor immune response. Radiotherapy triggers ATP release from dying tumor cells, resulting in activation of the NLRP3-ASC-inflammasome axis and subsequent secretion of IL-1β. Multiple cytokines and chemokines, reactive oxygen species, nucleic acids, lipids and amino acids, mitochondrial components, other cellular proteins, normally less visible to the immune system, are released by dying/dead cancer cells. Calreticulin exposure could differentiate immunogenic from non-immunogenic cell death, as well as the release of ATP and HMGB1, induced also in non-apoptotic cell death. Finally, IFN-γ and TNF-α, released by effector T cells, attract and activate other immune cells, including NK cells and macrophages, which detect and eradicate tumor cells [55-64].
Discussion
The antigenic profile of the dying cancer cells and the immunological composition of the TME, with cytokines, chemokines, metabolites, support cancer-specific T-cell immune response, thereby enhancing long-lasting tumor regression and promoting the immunological memory against malignant cells of the same type. Duration and nature of the contacts between cellular fragments and the immune system impact on activation versus inhibition of the above processes, enabling or disabling the TME to the execution of adaptive immune responses (ICD or inflammatory cell death). Autophagic cell death is caspase-independent and occurs without chromatin condensation, but with massive vacuolization. However, autophagy, often disabled in cancer, has been shown to be necessary for the induction of immunogenicity. Crosstalk between autophagy and apoptotic pathways occurs through shared molecules, such as p53 and p19ARF. Cytotoxic agents have been shown to induce necrotic cell death in apoptotic-defective tumor cells. The immunogenicity of necrotic cell death can fall in disproportionate inflammatory reactions, which are activated to mount protective immune responses. Alternative forms of regulated cell death are distinct from apoptosis by their characteristic lysis and the release of a wide range of intracellular components and inflammatory cytokines, which promote inflammation and/or direct a beneficial immune response [49, 54, 61]. Tumor lysis with cytokine release shares, at least in part, common pathogenetic mechanisms with autoinflammatory diseases, due to the similar activation and dysregulated release of cytokines, particularly IL-1β and IL-18. Knowledge of these diseases may provide useful insights into the development of new therapeutic strategies effective against CRS.
Monogenic autoinflammatory diseases are characterized by gain-of-function mutations in inflammasome sensors, which lead to excessive cytokine production and cell death. Gain-of-function mutations in pyrin are associated with the autoinflammatory disease Familial Mediterranean Fever (FMF) and drive excessive IL-1β release via GSDMD-mediated pyroptosis. TNF signaling is also involved. IL-1 inhibition with anakinra (a recombinant IL-1R antagonist), canakinumab (an anti-IL-1β monoclonal antibody), or rilonacept (an IL-1R/IL1-RA/IgG1 fusion protein decoy) improved outcomes in colchicine-resistant FMF. In atherosclerotic disease, plaque instability can derive from cell death, pyroptosis, and necrosis. In the CANTOS study (Canakinumab Antiinflammatory Thrombosis Outcomes Study), recurrent cardiovascular events were reduced by neutralizing IL-1β-mediated inflammation with canakinumab after myocardial infarction. Moreover, canakinumab unexpectedly reduced lung cancer incidence and mortality. IL-1-induced inflammation is thought to increase tumor invasiveness, growth, and metastasis. Excessive IL-18 release was observed in Behçet's disease, Hemophagocytic Lymphohistiocytosis with macrophage activation syndrome, and NLRC4 (NLR family CARD domain containing 4) -mediated autoinflammation with infantile enterocolitis. This subset of autoinflammatory diseases, associated with mutations in the pyroptosis mechanism, appears to be responsive to IL-18 blockade, but poor responsive to IL-1 blockade. Studies have shown that necroptotic signaling via RIPK3, MLKL, NLRP3, and caspase-1 can drive to the release of mature IL-1β, independently of GSDMD, through MLKL-mediated plasma membrane disruption, potassium ion efflux, and subsequent inflammasome-mediated maturation of IL-1β: this likely contributes significantly to the development of inflammatory diseases. Other studies have also hinted at an interaction between apoptotic and pyroptotic pathways, finding that caspase-8 can drive inflammasome activation. During infections or under conditions of TAK1 inactivation, multiple cell death pathways can be activated to prevent pathogen-mediated cell death and facilitate effective immune responses.
In multiple viral infections, the Z-DNA-binding protein 1 (ZBP1; also known as DAI), an inducible cellular DNA/RNA sensor with two Zα domains that bind pathogen-derived or cellular Z-DNAor Z-RNA, coordinates parallel cell death pathways of necrosis, apoptosis, and pyroptosis. In cancer, changes in apoptotic death pathways likely modify the form of cell death, that occurs downstream of TNF or other inflammatory signals. In the treatment of multifactorial diseases, such as inflammatory bowel disease, rheumatoid arthritis, and psoriasis, it is important to identify which steps in the process initiate and promote inflammation/hyperinflammation. The relative success of TNF blockade in treating many of these diseases suggests that TNF-induced inflammation and likely TNF-mediated apoptosis contribute to the acute state. Understanding whether TNF induces increased apoptosis, necrosis, pyroptosis, and cytokine release to promote disease may suggest alternative treatments. Proinflammatory cytokines can be also involved in promoting tumor growth, invasiveness and metastases, and ongoing studies are evaluating the role of MLKL and GSDMD in this context [65-68].
Conclusions
After a lethal insult, cells can trigger multiple overlapping signaling cascades, leading to cell death and consequent release of inflammatory cytokines. These processes, often resulting from anticancer therapies, but not only, must be interrupted early to avoid a fatal clinical outcome. At the same time, the potential loss of efficacy of such treatments, resulting from aggressive immunosuppression, must be considered. An additional, not insignificant, concern is the increased susceptibility to opportunistic infections. Tumor heterogeneity influences the treatment outcome, triggering cell death in some, but not all, tumor cells, and investigations on cell death in terms of immunogenicity have only just begun. Early identification of patients who will develop TLS and CRS syndromes is currently challenging. Circulating factors are estimated for their prognostic and predictive value in various oncological settings, while biomarkers predictive of response to immunotherapy and toxicity, particularly to ICD-inducing therapies in combination with ICIs, are still unknown. A fascinating topic is studying how dying cells can become more proactive in shaping anti-tumor immune responses altering the balance between antitumor tolerance and immunity through ICD manipulation. Insights, gained from studying the biological mechanisms of TLS and CRS and from the clinical use of corticosteroids and IL-6 blockade, have already improved patient management. The general therapeutic strategy for cytokine storm includes supportive therapies to preserve critical organ function, control the underlying disease, and eliminate the triggers for abnormal immune system activation, as well as targeted immunomodulation or nonspecific immunosuppression to limit collateral damage from the activated immune system.
However, with the increasing use of T-cell therapies, randomized clinical trials are urgently needed to improve the evidence-based approach to treating cytokine storm. For example, knowledge of endothelial dysregulation is lacking, despite its apparent central role in CRS. The mechanisms of neurotoxicity also deserve further investigation. The success of immunotherapy in various cancer types is leading to widespread application of this therapeutic strategy, and the incidence of TLS/CRS cases is expected to increase in the coming years. Advances in rheumatology in diagnosis and treatment of autoinflammatory diseases could inspire similar efforts in precision oncology: by deploying integrated "omics" analyses and artificial intelligence, specific targets of the hyperinflammatory circuitry could be identified and treated after precisely mapping the syndromes, resulting in more selective, effective, and safer therapy.
Conflicts of interest The Authors declare no competing interests.
Funding No funding was received.
References
- Lupuşoru G, Ailincăi I, Frăţilă G, et al. Tumor Lysis Syndrome: An Endless Challenge in Onco-Nephrology. Biomedicines 10 (2022): 1012.
- Fajgenbaum DC, June CH, et al. Cytokine Storm. New England Journal of Medicine 383 (2020): 2255–2273.
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. Journal for Immunotherapy of Cancer 6 (2018): 56.
- Yiu HH, Graham AL, Stengel RF, et al. Dynamics of a Cytokine Storm. PLoS ONE 7 (2012): e45027.
- Veroniki AA, Hutton B, Stevens A, et al. Update to the PRISMA guidelines for network meta-analyses and scoping reviews and development of guidelines for rapid reviews: a scoping review protocol. JBI Evidence Synthesis 23 (2025): 517–526.
- Howard SC, Trifilio S, Gregory TK, et al. Tumor lysis syndrome in the era of novel and targeted agents in patients with hematologic malignancies: a systematic review. Annals of Hematology 95 (2016): 563–573.
- Drakos P, Bar-Ziv J, Catane R, et al. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. American Journal of Clinical Oncology 17 (1994): 502–505.
- Molyneux K, Beck-Esmay J, Koyfman A, et al. High risk and low incidence diseases: Tumor lysis syndrome. The American Journal of Emergency Medicine 98 (2025): 283–288.
- Zhang Q, Zu C, Jing R, et al. Incidence, clinical characteristics and prognosis of tumor lysis syndrome following B-cell maturation antigen-targeted chimeric antigen receptor-T cell therapy in relapsed/refractory multiple myeloma. Frontiers in Immunology 4 (2023): 1125357.
- Levstek L, Janžič L, Ihan A, et al. Biomarkers for prediction of CAR T therapy outcomes: current and future perspectives. Frontiers in Immunology 15 (2024): 1378944.
- Hande KR, Garrow GC, et al. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. The American Journal of Medicine 94 (1993): 133–139.
- Cairo MS, Bishop M, et al. Tumour lysis syndrome: new therapeutic strategies and classification. British Journal of Haematology 127 (2004): 3–11.
- Cairo MS, Coiffier B, Reiter A, et al. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome in adults and children with malignant diseases: an expert panel consensus. British Journal of Haematology 149 (2010): 578–586.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124 (2014): 188–195.
- Coiffier B, Altman A, Pui CH, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. Journal of Clinical Oncology 26 (2008): 2767–2778.
- Durani U, Hogan WJ, et al. Emergencies in haematology: tumour lysis syndrome. British Journal of Haematology 188 (2020): 494–500.
- Yildizhan E, Kaynar L, et al. Cytokine release syndrome. Journal of Oncological Sciences 4 (2018): 134–141.
- Rose-John S, et al. Interleukin-6 signalling in health and disease. F1000Research 9 (2020): 1013.
- Holbrook J, Lara-Reyna S, Jarosz-Griffiths H, et al. Tumour necrosis factor signalling in health and disease. F1000Research 8 (2019): 111.
- Al-Qahtani AA, Alhamlan FS, Al-Qahtani AA, et al. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Tropical Medicine and Infectious Disease 9 (2024): 13.
- Shen S, Shao Y, Li C, et al. Different types of cell death and their shift in shaping disease. Cell Death Discovery 9 (2023): 284.
- Kroemer G, Galassi C, Zitvogel L, et al. Immunogenic cell stress and death. Nature Immunology 23 (2022): 487–500.
- Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy 6 (2021): 128.
- Dhuriya YK, Sharma D, et al. Necroptosis: a regulated inflammatory mode of cell death. Journal of Neuroinflammation 15 (2018): 199.
- Gebremeskel S, Johnston B, et al. Concepts and mechanisms underlying chemotherapy-induced immunogenic cell death: impact on clinical studies and considerations for combined therapies. Oncotarget 6 (2015): 41600–41619.
- Ricci MS, Zong WX, et al. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 11 (2006): 342–357.
- Hu L, Chen M, Chen X, et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death and Disease 11 (2020): 281.
- Zhai J, Gu X, Liu Y, et al. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: an update review. Frontiers in Pharmacology 14 (2023): 1152934.
- Wang YJ, Fletcher R, Yu J, et al. Immunogenic effects of chemotherapy-induced tumor cell death. Genes and Diseases 5 (2018): 194–203.
- Troitskaya OS, Novak DD, Richter VA, et al. Immunogenic Cell Death in Cancer Therapy. Acta Naturae 14 (2022): 40–53.
- Jiao Y, Cao F, Liu H, et al. Radiation-induced Cell Death and Its Mechanisms. Health Physics 123 (2022): 376–386.
- Adjemian S, Oltean T, Martens S, et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death and Disease 11 (2020): 1003.
- Chen H, Han Z, Luo Q, et al. Radiotherapy modulates tumor cell fate decisions: a review. Radiation Oncology 17 (2022): 196.
- Sun Q, Hong Z, Zhang C, et al. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduction and Targeted Therapy 8 (2023): 320.
- Vafaei S, Zekiy AO, Khanamir RA, et al. Combination therapy with immune checkpoint inhibitors: a new frontier. Cancer Cell International 22 (2022): 2.
- De Silva M, Tse BCY, Diakos CI, et al. Immunogenic cell death in colorectal cancer: a review of mechanisms and clinical utility. Cancer Immunology, Immunotherapy 73 (2024): 53.
- Zhu Z, Xue X, Zhou G, et al. Novel EGFR/PI3K dual-targeting nanoparticles induce immunogenic cell death in bladder cancer. Journal for ImmunoTherapy of Cancer 10 (2022): 1358.
- Jackson NM, Ceresa BP, et al. EGFR-mediated apoptosis via STAT3. Experimental Cell Research 356 (2017): 93–103.
- Nishihara S, Yamaoka T, Ishikawa F, et al. Mechanisms of EGFR-TKI-induced apoptosis and strategies targeting apoptosis in EGFR-mutated non-small cell lung cancer. Genes (Basel) 13 (2022): 2183.
- Garrido G, Rabasa A, Sánchez B, et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. Journal of Immunology 187 (2011): 4954–4966.
- Zugasti I, Espinosa-Aroca L, Fidyt K, et al. CAR-T cell therapy for cancer: current challenges and future directions. Signal Transduction and Targeted Therapy 10 (2025): 210.
- Navab R, Futela P, Kumari V, et al. Advancing multiple myeloma immunotherapy: a review of chimeric antigen receptor T-cell and bispecific T-cell engagers in revolutionizing treatment. Iran Journal of Medical Sciences 50 (2025): 1–10.
- Edeline J, Houot R, Marabelle A, et al. CAR-T cells and BiTEs in solid tumors: challenges and perspectives. Journal of Hematology and Oncology 14 (2021): 65.
- Lin RA, Lin JK, Lin SY, et al. Mechanisms of immunogenic cell death and immune checkpoint blockade therapy. Kaohsiung Journal of Medical Sciences 37 (2021): 448–458.
- Arimoto KI, Miyauchi S, Liu M, et al. Emerging role of immunogenic cell death in cancer immunotherapy. Frontiers in Immunology 15 (2024): 1390263.
- Anderson NM, Simon MC, et al. The tumor microenvironment. Current Biology 30 (2020): R921–R925.
- Finley SD, Popel AS, et al. Effect of tumor microenvironment on tumor VEGF during anti-VEGF treatment: systems biology predictions. Journal of the National Cancer Institute 105 (2013): 802–811.
- Salminen A, et al. The role of the immunosuppressive PD-1/PD-L1 checkpoint pathway in the aging process and age-related diseases. Journal of Molecular Medicine (Berlin) 102 (2024): 733–750.
- Wu B, Zhang B, Li B, et al. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduction and Targeted Therapy 9 (2024): 274.
- Scharping NE, Delgoffe GM, et al. Tumor Microenvironment Metabolism: A New Checkpoint for Anti-Tumor Immunity. Vaccines (Basel) 4 (2016): 46.
- Xiao Y, Li ZZ, Zhong NN, et al. Charting new frontiers: Co-inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Translational Oncology 38 (2023): 101794.
- Almawash S, et al. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 17 (2025): 880.
- Sax A, May P, Enssle S, et al. Defects in the necroptosis machinery are a cancer resistance mechanism to checkpoint inhibitor immunotherapy. Journal for ImmunoTherapy of Cancer 13 (2025): e010433.
- Duan Q, Zhang H, Zheng J, et al. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends in Cancer 6 (2020): 605–618.
- Grazioli S, Pugin J, et al. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Frontiers in Immunology 9 (2018): 832.
- Cicchinelli S, Pignataro G, Gemma S, et al. PAMPs and DAMPs in sepsis: a review of their molecular features and potential clinical implications. International Journal of Molecular Sciences 25 (2024): 962.
- Bolourani S, Brenner M, Wang P, et al. The interplay of DAMPs, TLR4, and proinflammatory cytokines in pulmonary fibrosis. Journal of Molecular Medicine (Berlin) 99 (2021): 1373–1384.
- Mo J, Hu J, Cheng X, et al. The role of high mobility group box 1 in neuroinflammatory related diseases. Biomedicine and Pharmacotherapy 161 (2023): 114541.
- Cheng CK, Yi M, Wang L, et al. Role of gasdermin D in inflammatory diseases: from mechanism to therapeutics. Frontiers in Immunology 15 (2024): 1456244.
- Miao R, Jiang C, Chang WY, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56 (2023): 2523–2541.
- Chang P, Li H, Hu H, et al. The Role of HDAC6 in Autophagy and NLRP3 Inflammasome. Frontiers in Immunology 12 (2021): 763831.
- Chen J, Zhao Y, Liu Y, et al. The role of nucleotides and purinergic signaling in apoptotic cell clearance: implications for chronic inflammatory diseases. Frontiers in Immunology 5 (2014): 656.
- Xiao L, Zhang L, Guo C, et al. “Find Me” and “Eat Me” signals: tools to drive phagocytic processes for modulating antitumor immunity. Cancer Communications (London) 44 (2024): 791–832.
- Koppensteiner L, Mathieson L, Neilson L, et al. IFNγ and TNFα drive an inflammatory secretion profile in cancer-associated fibroblasts from human non-small cell lung cancer. FEBS Letters 599 (2025): 713–723.
- Nigrovic PA, Lee PY, Hoffman HM, et al. Monogenic autoinflammatory disorders: conceptual overview, phenotype, and clinical approach. Journal of Allergy and Clinical Immunology 146 (2020): 925–937.
- Hou C, Wang Z, Eichenberger V, et al. Dysregulation of inflammasomes in autoinflammatory diseases. Joint Bone Spine 92 (2025): 105903.
- Crossman D, Rothman A, et al. The Canakinumab Antiinflammatory Thrombosis Outcome Study trial – the starting gun has fired. Journal of Thoracic Disease 9 (2017): 4922–4925.
- Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1843 (2014): 2563–2582.