Viability of Medicines Regulatory Harmonization (MRH) Regional Programs in Advancing Medicines Access – Case of New Drug Applications for East Africa Community (EAC) MRH
John. M. Mwangi*, Teresa N. Makokha
1Bayer East Africa Ltd, Kenya
*Corresponding author: John. M. Mwangi, Bayer East Africa Ltd, P.O Box 30321 – 00100 GPO Nairobi - Kenya.
Received: 16 June 2025; Accepted: 23 June 2025; Published: 07 July 2025
Article Information
Citation: John. M. Mwangi, Teresa N. Makokha. Viability of Medicines Regulatory Harmonization (MRH) Regional Programs in Advancing Medicines Access – Case of New Drug Applications for East Africa Community (EAC) MRH. Fortune Journal of Health Sciences. 8 (2025): 639 -648.
Share at FacebookAbstract
Access to essential medicines remains a significant challenge for patients in Africa. This is attributable to, among other factors, overreliance on imports from other countries for essential medicines, poor healthcare infrastructure, and weak regulatory systems. This is more apparent in the case of communicable diseases of Malaria, HIV, and TB in addition to the rising non-communicable diseases burden whose medicines access has been affected by the inefficient regulatory systems. Realizing this need, African countries through the support of WHO and the donor community started initiatives to strengthen regulatory systems over the last decade with the view to bring Africa towards being at par with other countries and regions of the world. An example has been the 2009 Africa Medicines Regulatory Harmonization (AMRH) program started with the goal of harmonizing regulatory requirements across Africa through regional economic communities as interim building blocks. In East Africa, such an initiative was launched in 2012 resulting in multiple regulatory milestones being fulfilled to date. Past studies have assessed the utility of the process from a regulator’s perspective. The current study assessed the impact of EAC MRH from an industry perspective as a tool to accelerate medicines access. The study looked at retrospective data covering fifteen years in the company regulatory database showing approval timelines for new drug applications using the traditional/national procedures in comparison to the EAC MRH procedure. From the results, there was a reported reduction in regulatory timelines for new drug application authorizations among the member countries from a high of up to 17 months to 12 months on average. Additionally, the EAC MRH process was found to be predictable, transparent, and a platform to practice regulatory reliance, therefore, facilitating quicker access to health products and medicines for patients. From the findings, applicants are encouraged to explore the EAC MRH route as an alternative to the national regulatory procedures as one way to guarantee faster access to health products and medicines. It is also recommended to address the gaps in the national procedures that have been shown to delay regulatory decisions in the East Africa region. This report however did not cover other regulatory aspects such as the sustainability of the program, post-approval changes, GMP inspection, and vigilance which are not as well developed and which should be a focus for future work by industry and regulators.
Keywords
regulatory harmonization, medicines, access, Africa, disease burden
Article Details
Introduction
In most developing countries, access to essential health products and medicines remains a challenge. This is especially the case in Africa where communicable diseases of HIV, Malaria, Tuberculosis have continued to form the biggest portion of disease burden [1] . In addition to communicable disease, an increase in non-communicable disease burden in the sub-saharan Africa is observed, adding to the already existing and yet to be contained communicable disease burden with early projections that non-communicable diseases would overtake the communicable disease burden by year 2030 [2]. In this context, access does not only refer to availability but as well that the medicines are safe for use, of the right quality, and affordable. Some of the factors affecting access to medical and health technology products include lack of adequate local manufacturing capacity, financial constraints by respective governments, poor healthcare infrastructure including distribution channels, and more importantly the regulatory framework in the countries. This situation has become apparent with the recent disease outbreaks. With the Covid-19 pandemic threatening to overrun the existing healthcare infrastructure in most countries around the world and more so in the East Africa region with indirect health effects [3].
One of the factors attributed to this poor access to health products and medicines in Africa has been the poor medicines regulatory infrastructure in the region [4]. Among the concerns observed, is the time it takes to introduce new medicines and health technology products in the countries across the region. Besides, there are concerns about the effectiveness of product life cycle management once the products are introduced in the markets [5]. Various regulatory pathways have been introduced in the past to help address these challenges [6]. Accordingly it has been observed over time that harmonizing drug regulation has improved the effectiveness and efficiency of governments regulating medicines [7]. This is supported by the fact that drug manufacturing in a globalized world requires the concerted efforts of all countries and regions. With this, the pioneering regulatory bodies such as US FDA and European Union’s EMA have progressively become open to more partnerships and collaboration and introducing in the case European Union centralized systems of regulating medical products. African countries have over the last decade embarked on a journey towards setting and following one regulatory standard for medical products [8].
Medicines regulatory systems
Medicine and health products regulatory review and approval form one of the critical steps in the process of drug development. This happens after years of rigorous research and development in basic research, preclinical and clinical studies. This last step before the product is finally introduced to the patient population is the focus of the current study. This study takes to account the associated cost, expertise, and time in the regulatory process. Besides being integral in the introduction of a medicinal product to the patients, this step of drug development dictates to a great extent how the product remains in the market [9].
Medicines are extensively regulated, with their handling right from development to the final use being a subject of rigorous regulatory requirements. Regulation of medicines has been on a constant evolution over, usually occasioned by constant learning in the currently available practices. There have been efforts to harmonize regulatory requirements for medicines leading to the emergence of common regulatory guidelines and legislations targeted at shaping the regulatory environment [7]. While this approach, often as a response to the needs in the regulatory environment, has helped to ensure that safe and good quality, efficacious products reach the patients, the same growth in regulatory requirements could pose a risk to timely access to medicines. This calls for the need to have a careful balance between regulatory systems and the need to expedite the approval process without compromising access to medication for patients. Overall, failure to carefully offer effective regulation could be a precursor of substandard and potentially counterfeit products in the market [10, 11]. Scientific evidence based, transparent and efficient regulation of health products is important to ensure access to health products. Conversely, lack of adequate regulatory systems is a hindrance to access with negative impact on public health of the populations at risk of disease burden [11]. One way to assess the impact on access is the time lag between when a product becomes available in countries with well-developed regulatory systems to the time the product becomes available in the countries with developing regulatory systems. A period of up to 7 years has been observed [5]. This is often the case as manufacturers are put off by the unpredictable, fragmented approach observed in countries with weak regulatory systems which also happen to have the least economic incentives for pharmaceutical investments [12]. This has had an overall impact of denying early access to life saving health products to patients in countries that need them most [4]. One such way to arrive at science based, transparent and predictable efficient regulatory decisions has been the concept of regulatory harmonization and convergence [8]. In doing so, it would enable countries and regions to focus on value added activities as well as share work among them in an environment of mutual trust, thereby reducing the overall time required for individual countries to carry out individual regulatory reviews often with limited resources [6],[13].
The observed evolution in regulatory requirements has led to the development of standardized requirements such as those by the International Conference on Harmonization (ICH) originally led by Japan, EU and the USA. These guidelines while very good in safeguarding the integrity of medicines regulation, may be more useful for the drug development process in the more developed markets where safety is more of a primary concern compared to the cost [14]. As much as these requirements are being adopted by non-ICH members, the actual impact on medicines access is varied mostly because of the complexity that comes with the uptake of the more complex harmonized requirements. This skews the development of medicines and health products regulatory systems to the developed countries and regions and by extension may imply that the innovative products that require developed regulatory systems may only become available in the developing and low-income countries with significant delay. It is against this background that African countries, have sought to overcome the challenge of poor regulatory systems and limited medicines access by putting in place initiatives like the AMRH Program [8]. This program which is the forerunner of the Africa Medicines Agency (AMA) was conceptualized to be implemented through regional economic communities (RECs). In East Africa, the regional secretariat has well-established harmonized guidelines for the regulation of health commodities within the members states. [15], [16]. Over the last decade, WHO and other development partners have invested significantly towards building capacity among regulatory bodies in Africa with the view to reaching a higher standard of regulation. While there is still more work remaining to be done, significant steps have been covered as demonstrated by the establishment and support of joint assessment programs in the regional economic communities involving member countries such as the case in East Africa Community and the Southern African Development Community (SADC) region.
In the case of EAC region, the region reported a reduction in product evaluation timelines to a record of 8 months, down from the regular timelines of 24 months [16]. Besides, WHO has embarked on a systematic approach of benchmarking regulatory authorities across the world as a tool to assess progress in the regulatory system strengthening process [17]. In this case, the so-called Global Benchmarking Tools have been deployed to as well support regulatory bodies with respective institutional development plans [18], [19]. This has seen at least eight (8) countries in Africa achieve maturity level 3 with dozen others in the assessment process. This exercise will in the end support a more systematic process of driving regulatory systems strengthening among health authorities across the world and especially in Africa.
The Journey to one Africa under Africa Medicines Regulatory Harmonization (AMRH)
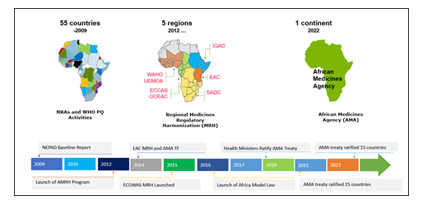
Africa Union through its NEPAD agency, has long supported efforts to develop strong medicines regulatory systems in line with World Health Assembly (WHA) resolution 67.20.[20], [21]. In an effort to reduce and potentially eliminate the fragmented nature of the medicines regulatory systems and requirements within the continent, the Africa Medicines Harmonization initiative was devised expected to progress as shown in Figure 1 below, a program supported by Africa Union and development agencies such as the World Bank, DFID among others in 2012 [6].
The launch of AMRH signaled a new dawn towards the events that we witness today. At the same time, efforts by WHO and other partners to support and lead Regulatory Systems Strengthening (RSS) activities continued to synergize the AMRH efforts [22]. In 2014, the idea to finally set up Africa Medicines Agency was first introduced. This would set in motion a series of parallel activities that culminated to the establishment of AMA treaty on 11th February 2019 and the endorsement of AMA treaty by AU Assembly [23]. With this, AMA is seen as the culmination of all AMRH activities of the last decade with the view to bring in synergies from among the countries National Medicines Regulatory Agencies (NMRAs), RECs-MRH initiatives and other continental working groups such as the Africa Vaccines Regulatory Forum (AVAREF) [8].
East Africa Medicines Regulatory Harmonization
EAC MRH was established as a response to the problem of hampered access to medicines in the region. It was reported that upon its initiation, as a system to assess generics drugs, there was initial uptake of the offered drug assessment procedures by innovator drug manufacturers and successful reduction in overall review timelines across EAC member states for products assessed and introduced to these countries down to 8 months from a baseline high of up to 24 months. Central to this was the earlier establishment of common technical guidelines that were in line with the global standards that allowed manufacturers to file applications in similar way they would in other countries around the world [16]. The practice of jointly assessing applications was started to support both capacity building and increase trust among partner states regulators and was shown to be very beneficial for the program. On the other hand, application of international standards during joint assessments implied that the standard of regulation was much higher that was the case in most member states, this may have had the initial effect of discouraging manufacturers who had less than state of the art dossiers and documentation. The work-sharing arrangement introduced peer review component among the assessors further sharpening the quality of assessments by the EAC MRH. This has had the tendency to give more confidence to the partner states to accept the assessment reports from the EAC joint assessment process [6], [15]. This has gained support from potential industry applicants who appreciate the predictability and standardization of the evaluation process. The EAC MRH has had additional benefits in the regulatory environment in the region as among other initiatives it has been shown to have triggered accelerated maturity for the partner states NMRAs, with some of the countries that originally had no national agencies establishing NMRAs since the inception of the EAC MRH [19]. These new entities have faced accelerated growth partly due to exposure to other competent agencies such as the WHO, Swissmedic among others which have been partners in capacity building for EAC MRH [17].
Facilitated regulatory pathways and regulatory reliance
As a departure from the past, the last decade has seen the emergence of alternative regulatory pathways [24], [25]. During the HIV pandemic, the need for medicines of high quality standard led among other things to the WHO prequalification program [26]. Out of this came the collaborative regulatory procedures which entailed the input of other regulatory authorities into the WHO prequalification scheme, itself a form of regulatory reliance [27], [28], [29].
With time and the onset of COVID 19 pandemic, there has been further refinement of facilitated regulatory pathways. In the case of South Africa, use of facilitated regulatory pathways that entailed abridged reviews and verification reviews have been shown to reduce regulatory review timelines by up to 68% among other benefits such as clearing of regulatory backlogs [30].
Conceptual Framework
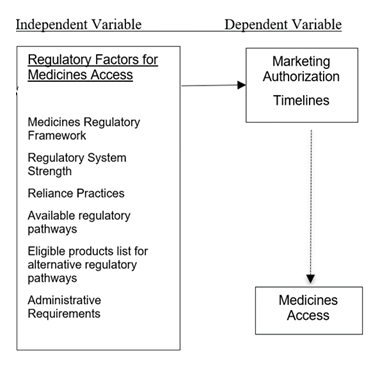
This study explored the case of using the state of medicines regulatory environment as a measure for medicines access in East Africa region. This study was based on the conceptual framework presented in the figure 2 below.
This framework seeks to demonstrate the factors that impact the medicines regulatory environment and measured by the approval lead times by applicants for new drug application in East Africa Region. These factors include the overall state of regulatory systems, use and availability of alternative regulatory pathways, criteria for using the various pathways such as the EAC MRH. It is expected that the above factors could have an impact on the overall efficiency of the EAC MRH program as reflected by industry perspective.
Summary
The reviewed literature has provided the details of the journey towards Africa Medicines Regulatory Harmonization as part of the wider, global effort to harmonize regulatory requirements for medicines and other health. The literature indicates the work so far done from regulators perspective and puts emphasis on the case of East Africa region which has been a pioneer for regulatory harmonization in Africa. The literature also identified areas of ongoing recommendations to make regulatory harmonization realize the full potential as driver for medicines access.
Methods
Research design
Descripto-explanatory study design was utilized implying a combination of explanatory and descriptive study method. This was important being the first of its kind done by a single organization and with an attempt to understand the EAC MRH program through detailed description of the procedure as a baseline for future reference by industry applicants without effecting any interventions to the procedure. The study was based on a combination of qualitative and quantitative methods. The qualitative aspects provided a contextual background while quantitative aspects addressed key performance indicator of approval lead times associated with the EAC MRH program compared to the national regulatory pathways.
Population
The study was carried out using information from one company as accumulated over a period of thirteen years, inclusive of the time since the inception of the EAC MRH program. All the applications filed and / or approved for the study company were used.
Data Collection and Analysis
The data required for the study was collected from the company’s internal Regulatory Information Management system (BRAVE System). This data also included engagements details with the EAC MRH, including through the trade associations engagements and internal company correspondences with EAC MRH program.
Limitations of the study
The scope of the study is limited to detailed assessment of experience by one company over a period and may therefore have limited inference across the industry. The framework for this study will nevertheless inform a systematic look into the effectiveness of the EAC MRH.
Results and Discussion
This chapter provides a discussion on the findings. The study company has been engaged in the EAC MRH initiative since the year 2012. The following has been the results and phases of this engagement.
Development of Guidelines and Operational Procedures
The period 2012 – 2014 was spent in development of key regulatory guidelines necessary for submissions to the EAC MRH program wherein the company presented the views in key main thematic areas of;
- Eligibility criteria of products to be approved through the EAC MRH program
- Handling of post-approval changes
- Handling of lifecycle activities – renewals and retentions
- Review and approval timelines
- Handling of GMP inspections
- Utilization of regional Information Management System
- Industry engagement
- Quality Management System
- Activities beyond new drug application
In the context of this study the “Compendium of Guidelines for Marketing Authorization of Human Medicinal Products” was the primary source of EAC guidelines. The specific ones considered included
- EAC Guidelines on Procedural Aspects for Applications for Marketing Authorization of Pharmaceutical Products
- EAC Procedure on Evaluation of Quality of Active Pharmaceutical Ingredient
- Infographic for EAC Joint Assessment Procedure
- EAC Guidelines on Stability Testing Requirements for Active Pharmaceutical Ingredients (APIs) and Finished Pharmaceutical Products (FPPs) )
- EAC Guidelines on Format and Content of Summary of Product Characteristics for Pharmaceutical Products
- EAC Guidelines on Format and Content of Labels For Pharmaceutical Products
- Guidelines for Submission of Documentation for Registration of Biotherapeutic Products
Following a series of industry and EAC Secretariat engagements, it became apparent that the system in place would not answer all questions, rather industry would have to do with incremental progress which was as well informed by the later to be developed 2016-2021 strategic plan by the EAC MRH. An example here was the lack of variation guideline which meant that industry would have to follow the new drug application procedure for marketing authorization application through EAC MRH program and thereafter revert to use of national variations guidelines while maintaining licenses in the individual countries. These industry engagements were done in a series of meetings between industry and EAC secretariat.
Utilization of EAC MRH for New Drug Application
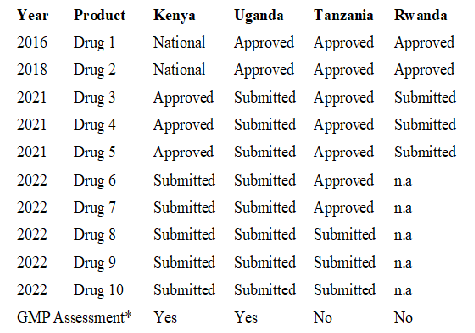
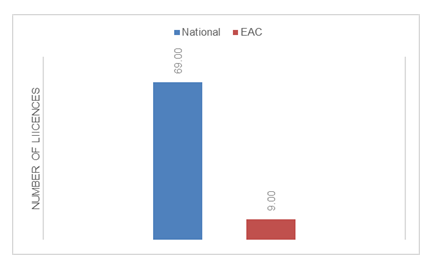
The company started the application of the EAC MRH process for New Drug Applications from the year 2016 with the results as shown in Figure 3 and Figure 4.
Note.
National means that the product was approved by the NRA after evaluation via national registration pathaway.
Approved means that the product was approved after evaluation by the EAC program.
Submitted means that the product has been submitted for registration via the EAC joint assesment process.
GMP Assessment* refers to certification in the individual countries.
Drugs assessed were in the oncology, ophthalmology and cardiology classes - all of them being innovator products and with EMA approval.
Product: Drugs did not fit the initial eligibility criteria but there was flexibility by EAC MRH to make the assessments
Kenya: Reported delays in issuance of final registration Preregistration analysis delays the final approval 90 days approval requirement not followed
Uganda: Unclear procedure post joint assessment report
Tanzania: Seamless process from joint assessment report with automatic approval within the 90 days rule
Rwanda: Limited exposure since the agency is recently established in (2020)
Note: *Total for each country of both EAC and National timelines
** National Timelines
*** EAC Timelines
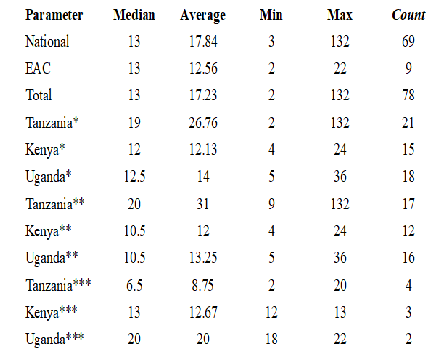
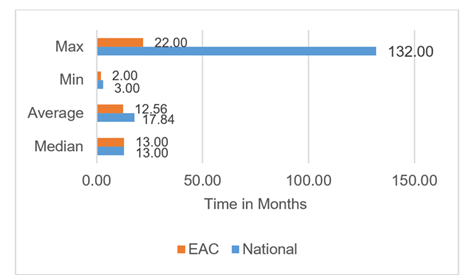
The median approval time for overall registration timeline was found to be 13 months (n=78), with no observed change between the national (n=69) and the EAC-MRH (n=9) procedure (Figure 5). EAC MRH procedure had an overall mean time to approval at 12.56 months compared to more than 17 months for the national procedure. Comparing the three main EAC countries, Tanzania has the longest overall average approval timelines at 26.76 months (n=21), with Kenya at 12.12 months (n=15) and Uganda at 14 months (n=18). However with the EAC MRH procedure, Tanzania reported lowest average at 8.75 months (n=4) followed by Kenya at 12.67 months (n=3) with Uganda coming last at 20 months (n=2). The national procedure in Kenya has a slightly shorter period at 12 months (n=12), followed by 13.25 months (n=16) with Tanzania having the highest average at 31 months (n=17). In Tanzania, the EAC MRH compares more favorably at 8.75 months average approval timelines vs the national procedure at 31 months, while as in Kenya there is little difference between the EAC MRH and the national procedure at 12.67 and 12 months respectively with a slight increase in timelines under EAC MRH procedure. In Uganda, the national procedure takes on average 13.25 months compared to the much longer EAC MRH procedure at 20 months.
Minimum approval timelines of 2 months and 3 months were reported for national and EAC MRH respectively. A high of 132 and 22 months were reported for the national and EAC MRH procedure respectively (Figure 6).
Discussions
In the overall data collected over the period 2010-2023, it was observed that the EAC procedure only kicked in 2017 meaning fewer number of application (n=9) being fewer than the national procedure (n=69) across the selected countries in Eastern Africa Region. During this time, the average approval timelines for EAC MRH based applications was reduced to 12.56 months down from 17.84 months. This is a good reflection of the improvements made by the EAC MRH procedure. Assessing the timelines between the three countries in detail reveals that Tanzania has had the greatest benefit from the EAC MRH with the reported overall average lead time reduction from by more than 22 months. Uganda on the other hand has not benefited from EAC MRH procedure as shown by the average increase in overall approval timelines between national and EAC MRH with an increase of more than 6 months for the assessed licenses. Kenya on the other hand has a slight declined its approval timelines even with introduction of EAC MRH procedure. Tanzania being the lead agency for medicines evaluation, has had a steady reduction in approval lead times compared to other EAC member states of Kenya and Uganda. There are some explanations as to the rather positive outcomes observed in Tanzania and which may serve as learning points for other countries in and outside of EAC region. It is worth noting that Tanzania has recently received the WHO ML 3 status which has come with more strengthening of the regulatory authority in the country [17]. In addition, the implementation of the e-platform for application filing has made it more efficient for applicants to interact with the health authority in Tanzania with an overall reduction in time lost through the application process. The regular publishing of review milestones in the e-platform has supported timely responses by the applicants helping to cut down on the overall approval lead times. The seamless transition from the positive opinion report to health authority approval in Tanzania has also reduced time lost in the transition from the EAC MRH report to achieving marketing authorization during the allowed 90 days window with some applications receiving approvals within the first 30 days. In Kenya, the EAC MRH procedure has now had significant improvements in the overall approval lead times. Perhaps a testament to the fact that in the period under study, most of the products had already been subjected to the national procedure in Kenya which is the lead market for product introduction in the East Africa region. Kenya has now established a pathway for EAC MRH filed applications and commits to follow the 90-day rule.
In the past, this has not worked perfectly due to the long list of products in the national system which is yet to be fully streamlined as is the case currently in Tanzania. It is expected that with Kenya aspiring for ML 3 accreditation, there will be better clarity on the exact path followed by the EAC MRH-based products, which will allow the country to take full advantage of the expedited review timelines in EAC MRH. It is worth noting that Kenya has had an evolving e-platform that has helped streamline applications including designating a pathway in the system for the EAC MRH-based products. This will continue to support the streamlining of the national review process. Kenya is among the few countries that still has additional administrative requirements for example, the requirement for in country sample analysis. While this may still be addressed in parallel with the application process, the time taken and complexity associated especially with newer non-pharmacopeia substances analysis serves to increase the entire marketing authorization timelines. In the case of Uganda, there has not been a designated EAC MRH procedure in the national authority, nor is there an e-platform for the health authority to work with. As such, there is an unclear pathway to achieving marketing authorization following the receipt of a positive opinion report from the EAC MRH Joint Assessment session. This can partly explain why there is a lag in the receipt of marketing authorization in Uganda compared to other EAC member states and consequently the overall long lead times reflected in Uganda.
Conclusion
Despite differences among the EAC member states, EAC MRH program has had a positive impact in promoting access to medicines in the region. Overall, there have been improvements in the approval timelines for new drug applications. Tanzania has had the highest impact with Kenya and Uganda not fully taking advantage of the process to support faster access to health products and medicines in the respective countries. The use of reliance practices has played a big role in ensuring expedited review and approval of the submitted products. Additionally, the process has evolved over time with major improvements witnessed in Tanzania where timelines have been on steep decline.
Recommendations for Next Steps
From the above findings, it is observed that the EAC MRH process for marketing authorization by applicants has had a positive impact on the overall regulatory timelines and subsequently for the medicines access to patients in East Africa region. This benefit however is not very apparent and is pegged on having the right structure and systems in the respective countries. These include the presence of
- A designated regulatory pathway that is dedicated to EAC MRH-assessed products. This designated process is not only a show of commitment from the respective country but also acts to provide the necessary transparency to the applicants who wish to follow the EAC MRH pathway.
- The list of products eligible for the EAC MRH products is currently best suited for the products that have been recently approved by stringent regulatory authorities as the EAC MRH is applying a reliance mechanism while making assessments of dossiers The above timelines reflect the adoption of reliance practices. It is important to note that the above timelines vary from product to product and takes into consideration whether the product is already approved in other jurisdictions. In the case of generics medicines, the requirement for additional data including bioequivalence studies may remain a hindrance, especially to regional local manufacturers. As such, there is the need to make the process robust enough to also support local and regional generic manufacturers to use this regulatory pathway to obtain marketing authorization for generic drugs.
- In addition to the transparent process in place indicating the timetable of assessment meetings and the TMDA (Tanzania Health Authority) e-platform that allows applicants to track their applications step by step, there is a need to implement similar transparency mechanisms in other East African countries as one means to help them make full use of this pathway towards accelerating regulatory decisions.
- The scope of activities covered by this study is limited to initial marketing authorization applications. While the EAC MRH process is keen to extend the set of activities to other regulated areas such as market surveillance, it is important to note that the extent of development of other regulatory activities is still lagging that of marketing authorization. The case of post-approval changes is particularly important to note. Handling of post-approval changes is not subject to the EAC MRH variation guideline but at the national level. Considering the unique country requirements and approval timelines already highlighted, there is the danger that the registered drug product dossier starts to differ between the countries thereby defeating the purpose of this procedure. It is therefore imperative that there is a concerted effort to bring the handling of variations for centrally assed products to the scope of the EAC MRH joint assessment.
It is recommended that the countries of Kenya and Uganda streamline the national procedures to be in sync with the EAC MRH program and take advantage of the faster review timelines achieved at EAC MRH to guarantee early entry and access of medicines in the two countries. Rwanda still needs to fine tune the process.
In addition to the new drug application process, the EAC MRH should expand the scope of activities to offer a holistic approach especially in handling of post approval changes. Finally, due to the different timelines at the EAC level and the subsequent national last mile process for issuance of marketing authorization, future studies should delve into disaggregating the full approval timelines and provide insights about the time taken at the EAC joint assessment process and that spent by the national agencies to issue marketing authorization to applicants.
Ethics approval and consent to participate.
Not required
Consent for publication
Consent for publication was provided by the study organization.
Competing interests
The authors declare that they have no competing interest.
Acknowledgements
We would like to thank my company for allowing me time off work to work on this project as well as supporting my access to the data used in this study.
References
- Amref, “Communicable diseases.” [Online].
- C Kraef, et al., “Fighting non-communicable diseases in East Africa: assessing progress and identifying the next steps,” BMJ Glob Health 5 (2020): e003325.
- E Barasa, J Kazungu, S Orangi, et al. “Indirect health effects of the COVID-19 pandemic in Kenya: a mixed methods assessment,” BMC Health Serv Res 21 (2021): 740.
- K Narsai, A Williams, and AK. Mantel-Teeuwisse, “Impact of regulatory requirements on medicine registration in African countries - perceptions and experiences of pharmaceutical companies in South Africa,” South Med Rev 5 (2012): 31–37.
- V Ahonkhai, SF Martins, A Portet, et al. “Speeding Access to Vaccines and Medicines in Low- and Middle-Income Countries: A Case for Change and a Framework for Optimized Product Market Authorization,” PLoS ONE 11 (2016): e0166515.
- H Sillo, et al. “Coming together to improve access to medicines: The genesis of the East African Community’s Medicines Regulatory Harmonization initiative,” PLoS Med 17 (2020): e1003133.
- Vogel D. “The Globalization of Pharmaceutical Regulation,” An International Journal of Policy and Administration 11 (1998).
- M Ndomondo-Sigonda, J Miot, S Naidoo, et al. “The African Medicines Regulatory Harmonization Initiative: Progress to Date,” MRAJ 6 (2018).
- NB Agarwal and M Karwa. “Pharmaceutical Regulations in India,” in Pharmaceutical Medicine and Translational Clinical Research, Elsevier (2018): 215–231.
- UNIDO, “Pharmaceutical Sector Profile: Kenya Global UNIDO Project: Strengthening the local production of essential generic drugs in the least developed and developing countries.” UNIDO (2010).
- L Roth et al. “Expanding global access to essential medicines: investment priorities for sustainably strengthening medical product regulatory systems,” Global Health 14 (2018): 102.
- Kamwanja L, Saka J, Ndomondo-Sigonda M, et al. “Situational Analysis Study on Medicines Registration HarmonisationinAfrica: Final Report for the East African Community.” NEPAD (2010).
- L Roth et al. “Expanding global access to essential medicines: investment priorities for sustainably strengthening medical product regulatory systems,” Global Health 14 (2018): 102.
- P Trouiller, P Olliaro, E Torreele, et al. “Drug development for neglected diseases: a deficient market and a public-health policy failure,” The Lancet 359 (2002): 9324.
- JM Mwangi, “Towards African Medicines Regulatory Harmonization: The case of the East African Community,” PPL 18 (2016): 1–4.
- JH Mashingia et al. “Eight years of the East African Community Medicines Regulatory Harmonization initiative: Implementation, progress, and lessons learned,” PLoS Med 17 (2020): e1003134.
- A Khadem Broojerdi, H Baran Sillo, R Ostad Ali Dehaghi, et al. “The World Health Organization Global Benchmarking Tool an Instrument to Strengthen Medical Products Regulation and Promote Universal Health Coverage,” Med 7 (2020): 457.
- Narendran Narendran and MP Venkatesh. “WHO Global Benchmarking Tool (GBT) For Evaluation Of National Regulatory Systems,” IJPR 12 (2020).
- J Guzman, EO’ Connell, K Kikule and T Hafner. “The WHO Global Benchmarking Tool: a game changer for strengthening national regulatory capacity,” BMJ Glob Health 5 (2020): 8.
- Africa Union – PMPA. “PHARMACEUTICAL MANUFACTURING PLAN FOR AFRICA.” Africa Union (2012).
- WHO, “Regulatory system strengthening for medical products - World Health Assembly (WHA) resolution (2014): 67.20.
- BM Ncube, A Dube, and K Ward. “Establishment of the African Medicines Agency: progress, challenges and regulatory readiness,” J of Pharm Policy and Pract 14 (2021): 29.
- M Makoni. “African Medicines Agency to be established,” The Lancet 398 (2021): 10310.
- A Keyter, S Salek, N McAuslane, S Banoo, et al. “Implementation of a Framework for an Abridged Review Using Good Reliance Practices: Optimising the Medicine Regulatory Review Process in South Africa,” Ther Innov Regul Sci 54 (2020): 1199–1207.
- Luigetti, Riccardo, Bachmann, Peter, et al. “Collaboration, not competition: developing new reliance models,” WHO Drug Information; Geneva 30 (2016): 558–566.
- EFM ‘t Hoen, HV Hogerzeil, JD Quick, et al. “A quiet revolution in global public health: The World Health Organization’s Prequalification of Medicines Programme,” J Public Health Pol 35 (2014): 137–161.
- A Vaz et al. “WHO collaborative registration procedure using stringent regulatory authorities’ medicine evaluation: reliance in action?,” Expert Review of Clinical Pharmacology 15 (2022): 11–17.
- M Caturla Goñi. “Accelerating regulatory approvals through the World Health Organization collaborative registration procedures,” PPL 18 (2016): 1–4
- Lori Alquier “Regulatory Harmonization in a Resource-Limited Setting: The World Health Organization Collaborative Procedure for Accelerated Registration,” Aug (2021).
- A Keyter, S Salek, L Danks, et al. “South African Regulatory Authority: The Impact of Reliance on the Review Process Leading to Improved Patient Access,” Pharmacol 12 (2021): 699063.