Vinegar Production by the Static Fermentation of Subcritical Water - Treated Mixtures of Sake Lees and Rice Bran
Shoji Hirayama1,2, Sae Namba5, Takachika Hoshino2,3, Yuriko Hoshino2,3,4, Kazuharu Yamato2,6, Munehiro Hoshino6, Mitsuru Sasaki7,8
1Graduate School of Science and Technology (GSST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
2M&A Food Technology and Biology of Technical Center (M.A.F.T), 2349 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
3Agri Co LTD, 2349 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
4Maruboshi Vinegar Inc, 2420-1 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
5Department of Materials Science and Applied Chemistry, Faculty of Engineering, Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
6Maruboshi Vinegar Co LTD, 2425 Tabara, Kawasaki-Machi, Tagawa-Gun, Fukuoka 827-0004, Japan
7Institute of Industrial Nanomaterials (IINa), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
8Faculty of Advanced Science and Technology (FAST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
*Corresponding Author: Mitsuru Sasaki, Institute of Industrial Nanomaterials (IINa), Kumamoto University, and Faculty of Advanced Science and Technology (FAST), Kumamoto University, 2-39-1 Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
Received: 16 September 2025; Accepted: 23 September 2025; Published: 16 October 2025
Article Information
Citation: Shoji Hirayama, Sae Namba, Takachika Hoshino, Yuriko Hoshino, Kazuharu Yamato, Munehiro Hoshino, Mitsuru Sasaki. Vinegar Production by the Static Fermentation of Subcritical Water - Treated Mixtures of Sake Lees and Rice Bran. Journal of Food Science and Nutrition Research. 8 (2025): 98-106.
DOI: 10.26502/jfsnr.2642-110000180
Share at FacebookAbstract
Sake lees and rice bran are major by-products of the Japanese food industry. As these by-products are discarded in large quantities, sustainable approaches to utilize these feedstocks are needed. We therefore investigated the production of vinegar through static fermentation using a mixture of subcritical water–treated sake lees and rice bran. Subcritical water treatment was previously shown to promote the liquefaction of these by-products to generate solutions rich in amino acids and minerals. In this study, we prepared subcritical water–treated sake lees and rice bran solutions at various mixing ratios and examined the effect on vinegar production. The amino acid and mineral content of the vinegar varied depending on the mixing ratio, and the higher the mixing ratio of subcritical sake lees solution, the higher the amino acid concentration, and the higher the mixing ratio of subcritical rice bran solution, the higher the carbohydrate and mineral content. This study proposes a method for producing vinegar with high nutritional value comparable to conventional products by reusing by-products from the Japanese food industry.
Keywords
Subcritical water, Liquefaction, Rice bran, Sake lees, Vinegar
Subcritical water articles; Liquefaction articles; Rice bran articles; Sake lees articles; Vinegar articles
Article Details
Introduction
The Japanese food industry generates approximately 13 million tons of food waste annually. Although the amount of food wasted annually by the restaurant industry has decreased since the COVID-19 pandemic, the total amount of waste generated by food manufacturers has remained constant. Waste from the food industry accounts for approximately 86% of the total amount of food waste [1], indicating significant challenges in reducing this waste.
The Food Recycling Law of Japan promotes the utilization of food waste and has prompted efforts to develop a more circular and decarbonized society. Recycling and related measures throughout the Japanese food industry amounted to 87% in 2021 [2]. The authors of the present study have focused on brewing vinegar using food waste. Vinegar production generates large amounts of sake lees and rice bran, which are rather expensive to dispose of. In a previous study, we used subcritical water treatment to liquefy sake lees and rice bran. Analysis of the treated liquids revealed abundant amino acids and minerals [3]. Fermentation of these subcritical treatment liquids generated vinegar similarly rich in amino acids and minerals, suggesting the possibility of producing a new type of vinegar with higher nutritional value than conventional products [4,5].
Two notable characteristics make subcritical water useful for a wide range of applications, such as hydrolysis and sample extraction [6,7]. First, the dielectric constant of subcritical water decreases with increasing temperature. For example, at 200–300°C, the dielectric constant of water is similar to that of various polar solvents. Second, the large ion product of subcritical water suggests that it exhibits properties of both acid and base catalysts.
A number of previous studies have examined the use of subcritical water for extracting valuable materials from food waste. For example, Gaecia (2006) examined the recovery of catechins and proanthocyanidins from grape seeds [8], whereas Yoshida (2006) studied the recovery of various amino acids and oils, such as DHA, by decomposing waste from the fish industry [9]. Furthermore, Domoto et al. applied subcritical water treatment to carbohydrate-rich breads and cakes and protein-rich lunchbox residues and showed that the resulting solids were rich in amino acids and organic acids. The solid material was then applied as compost for strawberries [10]. Miyashita et al. used subcritical water to extract the bitter components of green tea, thereby reducing the unpleasant aspects of taste compared with ordinary green tea. They also reported that tea prepared using subcritical water treatment contained more useful components than tea prepared using ordinary hot water extraction [11]. Finally, Hazwani et al. applied subcritical water treatment to oil palm trunk (OPT) biomass to produce solid biochar. They suggested that disposed OPT biomass could be utilized as an alternative fuel or soil improvement material [12].
Several prior studies have focused on sake lees and rice bran, which are also the targets of our research. Wakita (2005) reported that treating sake lees with subcritical water or supercritical carbon dioxide increased the soluble components to approximately 70% [13]. Adachi (2009) attempted the subcritical extraction of defatted rice bran and reported that the concentrations of sugars and proteins in the extract increased with extraction temperature up to 200°C [6]. The use of subcritical water thus holds promise as a greener alternative to traditional organic solvent extraction methods [24,25].
We previously conducted acetic acid fermentation using a 1:1 mixture of liquefied sake lees and rice bran after treatment with subcritical water and found that both feedstocks retained high concentrations of nutrients and did not interfere with each other when mixed and extracted. This indicated the possibility of producing vinegar with a nutritional value equivalent to that of current commercial products [5]. Here, we describe a detailed analysis of vinegar production using subcritical water treatment of sake lees and rice bran and of the amino acids and minerals in the resulting vinegar. We varied the mixing ratio of sake lees and rice bran with water prior to acetic acid fermentation. Our aim was to determine whether vinegar can be efficiently produced by selectively utilizing the characteristic valuable components of sake lees and rice bran, thereby contributing to the development of a sustainable and value-added approach for recycling food industry by-products.
2 Experimental
2.1 Raw materials and standard reagents
2.1.1 Raw materials
The raw materials used in this study (rice bran and sake lees) were supplied by Maruboshi Vinegar Co. (Fukuoka, Japan). The appearance and chemical composition of rice bran and sake lees are presented in figure 1 and table 1, respectively.
|
Constituent |
Rice bran |
Sake lees |
|
Water content [%] |
9.69 |
56.2 |
|
Total nitrogen [ppm] |
1900 |
2510 |
|
Water solubility amino acids [ppm] |
43.6 |
127.1 |
|
Total organic carbon [ppm] |
8.4 |
8.8 |
|
Ammoniac nitrogen [ppm] |
4.2 |
21.3 |
|
Minerals [ppm] |
23.9 |
96.5 |
Citation for sake lees: reference [4]
Table 1: Overview of the major chemical constituents of rice bran and sake lees.
2.1.2 Compliance with regulations for by-product (sake lees and rice bran) use
The sake lees and rice bran used in this study were both by-products generated during our food manufacturing process (Maruboshi Vinegar Co), and the transfer and use of these by-products complied with all relevant laws, regulations, and guidelines, including the Waste Disposal and Public Cleansing Law (Japanese Law No. 137 of 1970) and the Nagoya Protocol (effective October 12, 2014).
2.1.3 Standard reagents
Type H amino acid mixture standard solution was used in this study, with calibration certified through measurement of primary standards, in accordance with Japan’s Measurement Law. Type H amino acid mixture standard solution was obtained from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan)
Standard reagents containing ammonium nitrogen, calcium, magnesium, potassium, and sodium were obtained from FUJIFILM Wako Pure Chemical Corporation. All calibrations conformed to Japan’s Measurement Law and were traceable to national reference standards.
2.2 Subcritical water treatment
Sake lees and rice bran obtained from Maruboshi Vinegar Co., Ltd. (Fukuoka, Japan) were employed for subcritical water processing. Details regarding the equipment setup are available in prior studies [3-5].
A 500-mL, SS316 stainless steel reactor (OM Labotec, MM500, Tochigi, Japan) was used for subcritical water treatment. Each run involved loading 45 g of sample and 300 mL of distilled water into the vessel, followed by agitation and sealing of the reactor.
A band heater was used to heat the samples to the appropriate target temperature: 120°C for 240 min (sake lees) or 180°C for 30 min (rice bran) [5]. These parameters were selected because they yielded the highest liquefaction efficiency in prior analyses.
|
Sample [g-wet] |
Residue [g-dry] |
Vol. recovered [mL] |
Liquefaction rate [%] |
Standard deviation [%] |
|
|
Sake lees |
45.2 |
26.4 |
332.5 |
77.1 |
1.53 |
|
Rice bran |
45.2 |
10.9 |
330 |
74 |
3.15 |
Table 2: Analysis of the liquefaction of sake lees and rice bran (n=6)
During subcritical water treatment, the internal reactor pressure ranged from 1.3 MPa to 2.6 MPa, primarily due to water vaporization and gas evolution. Upon treatment completion, the heating unit was detached, and the reactor contents were promptly cooled using a fan. The resulting mixture was subjected to suction filtration to separate the soluble filtrate from the solid residue. Key filtrate constituents were then quantitatively analyzed, including amino acids, nitrogen species, phosphorus, and essential minerals.
2.3 Analysis
This section provides an overview of the analytical procedures following subcritical water treatment, with a particular focus on the determination of the concentrations of amino acids, nitrogen compounds, phosphorus, and various minerals using established analytical techniques.
2.3.1 Amino acids
Amino acid analysis involved derivatization using 3-mercaptopropionic acid and O-phthalaldehyde, followed by separation using a 2.6-µm Kintex EVO C18 column (100 × 3 mm). Quantification was performed via high-speed liquid chromatography with fluorescence detection using a Nexera X2 column and SHIMADZU GLC system (Kyoto, Japan)
Shimadzu Corporation Amino Acid Analysis System
https://www.an.shimadzu.co.jp/products/liquid-chromatography/hplc-system/amino-acid-analysis-system/index.html
2.3.2 Total nitrogen concentration
Total nitrogen was measured according to the Kjeldahl digestion method [14].
2.3.3 Ammonia nitrogen concentration
Ammonia nitrogen levels were determined using the indophenol blue colorimetric method [15].
2.3.4 Phosphorus concentration
Phosphorus content was determined using the molybdenum-blue reaction method [16].
2.3.5 Mineral concentration
Minerals such as potassium, calcium, and magnesium were quantified using atomic absorption spectrophotometry on a model AA-7000 instrument (Shimadzu Corp.).
Shimadzu Corporation Elemental Analysis Base Paper Spectrophotometer
https://www.an.shimadzu.co.jp/products/elemental-analysis/atomic-absorption-spectroscopy/aa-7800/index.html
2.3.6 Liquefaction rate (%)
The liquefaction percentage was determined using the following equation, which accounts for the removal of moisture and residue from the raw material weight:
Liquefaction rate (%) = (Initial dry matter – Residue) × 100/Initial dry matter
The liquefaction rate was calculated by dividing the change in dry weight of the raw material before and after treatment by the dry weight of the raw material before treatment. This is the standard method for handling solid mixtures. Yamato et al. (2021) and Sasaki et al. (2003) also calculated the liquefaction rate using a similar method; thus, this calculation method was deemed appropriate for the present study.
2.3.7 Total organic carbon (TOC)
TOC was measured using a TOC analyzer (TOC-V SCH, Shimadzu, Kyoto, Japan). A 1-mL aliquot of liquefied sample was diluted to 50 mL with distilled water for analysis. TOC in the aqueous solution was calculated by subtracting the total amount of inorganic carbon (CO and CO2) dissolved in the aqueous solution (inorganic carbon: IC) from the total carbon (TC) obtained by catalytic combustion. This calculation method is described on the website of Shimadzu Corporation.
SHIMADZU Corporation "TOC Measurement Methods: TOC Academy"
https://www.shimadzu.com/an/products/total-organic-carbon-analysis/en/what/02.html
2.3.8 Protein concentration (g/100 mL)
Protein content was estimated based on total nitrogen as quantified using the Kjeldahl technique, applying a nitrogen-to-protein conversion factor of 6.25.
2.3.9 Lipid concentration (g/100 mL)
Lipid concentrations were determined using the liquid-liquid method [17].
2.3.10 Carbohydrate concentration (g/100 mL)
The carbohydrate concentration was estimated by subtracting the measured values of moisture, protein, lipid, and ash from the total mass.
2.3.11 Ash concentration (g/100 mL)
Ash content was assessed by incinerating the sample at high temperature, according to the direct ashing procedure outlined elsewhere [18].
2.3.12 Acidity (g/100 mL)
The acidity level was quantified via titration, as described elsewhere [19].
2.3.13 Caloric value (kcal/100 mL)
Energy content was calculated via the modified Atwater method, referencing standard coefficients for protein, lipid, and carbohydrate contributions [20].
2.4 Acetic acid fermentation of subcritical water–treated filtrate by static fermentation
As done previously, the filtrate was subjected to static fermentation to produce vinegar. Static fermentation is an ancient method still used by Japanese vinegar producers that requires no expensive equipment and allows small-scale production [21].
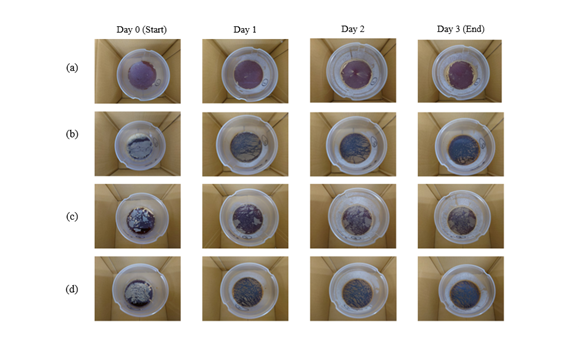
Subcritical water–treated sake lees and rice bran filtrates were mixed. The sake lees filtrate was treated at 120°C for 240 min, and the rice bran filtrate was treated at 180°C for 30 min. The sake lees filtrate to rice bran filtrate mixing ratios were 1:4 and 4:1 (each with a total volume of 600mL), respectively. These mixtures were inoculated with acetic acid bacteria (Acetobactor pasteurianus, analysis by Techno Suruga Lab), and the initial ethanol concentration and acidity were adjusted to 4% and 1.5% by the addition of appropriate amounts of 95% alcohol and 15% vinegar, respectively (Maruboshi Vinegar; Fukuoka, Japan). The mixtures were fermented statically at room temperature for 3 days, and fermentation was terminated when the ethanol concentration decreased to 0%. The samples were sterilized by heating to 85°C before analysis of the various components. Figure 2 shows the progress of static fermentation of four types of subcritical water–treated filtrate combinations. The far left portion of the figure shows the start of fermentation (day 0), and the far right portion of the figure shows the end of fermentation (day 3). The mixed vinegars indicate the sake lees to rice bran filtrate ratios. The white film visible in the figure is composed of acetic acid bacteria.
3. Results and Discussion
3.1 Static fermentation using each subcritical treatment mixture
Acetic acid fermentation was conducted by varying the mixing ratios of each subcritical treatment filtrate. The results are shown in figure 2. Panel (a) depicts the process of acetic acid fermentation of subcritical water–treated sake lees, panel (b) shows the fermentation of subcritical water–treated rice bran, panel (c) shows the fermentation process of a mixture of subcritical water–treated sake lees and rice bran filtrates at a ratio of 4:1, and panel (d) shows the fermentation of a mixture at a 1:4 subcritical water–treated sake lees to rice bran filtrate ratio.
All subcritical water–treated liquids became completely covered with a film of acetic acid bacteria as the fermentation progressed. On the third day of fermentation, the concentration of the added ethanol decreased to 0%, indicting the completion of fermentation.
The results shown in figure 2 suggest that both the activity of the acetic acid bacteria and the rate of bacterial membrane growth increase with increasing concentration of the subcritical water–treated sake lees filtrate. A high amino acid concentration, such as that in the subcritical water–treated sake lees filtrate, apparently enhances the activity of acetic acid bacteria. This is thought to result in more rapid completion of acetic acid fermentation, which would reduce costs when production is scaled up.
Table 3 shows the results of an analysis of general nutrients in vinegar produced by acetic acid fermentation of each subcritical water–treated sample. Each vinegar contained ≥4.2% acetic acid, meeting the Japanese standard for grain vinegar [22]. Acetic acid fermentation of subcritical water–treated filtrate thus conforms to the standards for vinegar, and the flavor and aroma of the products were similar to commercial vinegar. The results of our previous study suggested that changing the mixing ratio of subcritical water–treated sake lees and rice bran could alter the nutritional components of the resulting vinegar, as we here demonstrate.
Subcritical water–treated sake lees and rice bran were fermented at mixing ratios of 4:1 and 1:4. We observed that the higher the concentration of rice bran filtrate, the higher the amount of carbohydrate and ash (mineral components). The mixing ratio had little effect on the protein content, but the free amino acid concentration was higher for the 4:1 ratio sample.
The acidity standard for commonly sold grain vinegar (≥4.2%) was met, and this is not difficult in making vinegar. The more subcritical water–treated liquid is present in the rice bran, the more carbohydrates will be produced, and accordingly, the higher the energy value. This could be attributed to the high content of carbohydrate originally present in rice bran, but it was thought that the amount of carbohydrate, a nutritional component, could be altered by changing the sake lees to rice bran mixing ratio.
|
Energy (kcal) |
Protein (g) |
Lipid (g) |
Carbohydrate (g) |
Ash (g) |
Acidity (g) |
|
|
Sake lees: Rice bran (4:1) |
30±0.8 |
0.6±0.05 |
0.0±0 |
7.9±0.1 |
0.1±0.04 |
4.3±0.05 |
|
Sake lees: Rice bran (1:4) |
42±0.9 |
0.6±0.05 |
0.0±0 |
11.0±0.12 |
0.3±0.05 |
4.6±0.05 |
|
Sake lees |
28±0.8 |
0.6±0.05 |
0.0±0 |
7.6±0.1 |
0.1±0.04 |
4.9±0.05 |
|
Rice bran |
45±0.8 |
0.6±0.05 |
0.0±0 |
11.9±0.12 |
0.4±0.05 |
4.66±0.05 |
|
Grain vinegar (product) |
21±0.7 |
0.2±0.05 |
0.0±0 |
6.2±0.1 |
0.7±0.04 |
4.26±0.05 |
|
Brack vinegar (product) |
18±0.9 |
0.8±0.05 |
0.0±0 |
4.8±0.1 |
0.1±0.05 |
4.35±0.05 |
Values are expressed as the mean ± SD
Table 3: Nutritional analysis of prepared vinegars (per 100 mL; n=3)
3.2 Analysis of minerals and amino acids
3.2.1 Comparison of mineral content
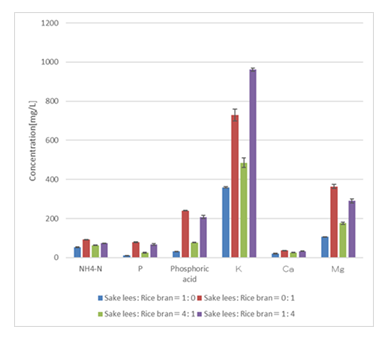
The results of an analysis of minerals in each vinegar (Figure 3) show that all of the vinegars contained high levels of potassium and magnesium. This is most likely because potassium and magnesium are relatively highly soluble minerals that readily dissolve in water in the subcritical water treatment solution. These minerals play an important role in physiological activity and are thus expected to be beneficial.
3.2.2 Comparison of essential amino acids
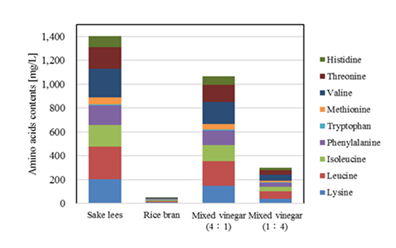
Figure 4 shows the results of a comparison of the content of essential amino acids in each vinegar. The amount of free essential amino acids increased as the proportion of subcritical water–treated sake lees filtrate or the mixing ratio of subcritical water–treated sake lees filtrate increased, suggesting that fermentation of subcritical water–treated sake lees filtrate produces vinegar with a larger amount of free amino acids.
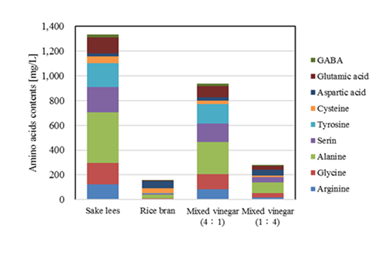
Figure 5 shows the results of a comparison of the content of non-essential amino acids and GABA in each vinegar. Again, the amounts tended to increase as the concentration of subcritical water–treated sake lees filtrate increased. The subcritical water–treated solution derived from sake lees had a higher free amino acid content than the subcritical water–treated solution derived from rice bran. This is thought to be due to differences in the composition and structure of proteins and peptides contained in sake lees and rice bran, which in turn affected the hydrolysis efficiency and amount of free amino acids produced through subcritical water treatment. Peptides and other substances may have accumulated in sake cake during the early stages of the fermentation process due to the activity of koji mold and yeast [26]. By contrast, rice bran contains a variety of functional components but is unfermented, so the amount of free amino acids newly generated by subcritical water treatment is considered to be relatively low [27]. These data thus suggest that subcritical water treatment using sake lees as a raw material is useful as an efficient method for generating free amino acids.
Table 4 shows the concentrations of aspartic acid and glutamic acid, which tended to decrease at the end of each fermentation. Aspartic acid decreased from 392.9mg/L in the sake lees filtrate to 22.1mg/L in the corresponding vinegar and from 127.3mg/L to 60.4mg/L in the subcritical water–treated rice bran vinegar. Glutamic acid decreased from 192.8mg/L in the sake lees filtrate to 129.8mg/L in the corresponding vinegar. These findings are consistent with those of a study by Nanba et al. (1985) [23], which showed that acetic acid bacteria utilize free amino acids during growth and fermentation. Both aspartic acid and glutamic acid are readily utilized as a nitrogen source for microbial growth and energy acquisition.
In the future, it will be necessary to examine the effects of the decrease in amino acid concentrations on flavor and taste components [28]. Future correlation analyses involving other amino acid and organic acid compositions, as well as changes in the microbial community structure, such as acetic acid machines, should help elucidate the mechanism in more detail.
|
Sake lees vinegar |
Rice bran vinegar |
Mixed vinegar (4:1) |
Mixed vinegar (1:4) |
Sake lees |
Rice bran |
|
|
Aspartic acid |
22.1±0.72 |
60.4±1.19 |
28.2±1.01 |
46.2±1.08 |
392.9±0.61 |
127.3±1.17 |
|
Glutamic acid |
129.8±0.76 |
4.2±0.47 |
87.3±1.04 |
32.4±0.51 |
192.8±0.93 |
2.7±0.76 |
Values are expressed as the mean ± SD
Sake lees: sake lees after subcritical water treatment
Rice bran: rice bran after subcritical water treatment
Table 4: Comparison of aspartic acid and glutamic acid concentrations before and after acetic acid fermentation (mg/L; n=3)
4. Conclusions
This study demonstrated the production of nutritionally rich vinegar through the static fermentation of subcritical water–treated sake lees and rice bran. Adjusting the mixing ratio of the treated lees and bran filtrates allowed for control of the nutritional profile of the product vinegar to increase the amino acid and mineral levels as desired. The proposed approach will facilitate better management of industrial food wastes and provide a high-value-added product.
The research described in this paper is still at the laboratory level. In recent years, interest in healthy eating in Japan has increased, especially with regard to functional ingredients. The vinegar produced as described in this paper contained the same or more useful components as commercially available vinegar. Therefore, in order to industrialize the process, further experiments to ensure reproducibility are required. In this study, batch-type subcritical water treatment was adopted as the treatment method, but in the future, in addition to this method, experiments will focus on the use of continuous-type subcritical water treatment, which can more efficiently reduce the volume of biomass. The primary goal of these process development studies is to expand the scale of production beyond what is now possible
Acknowledgments
This research was carried out with the support of the Graduate School of Science and Technology (GSST), Kumamoto University, and the M&A Food Technology and Biology Technical Center (MAFT).
Author contributions
Shoji Hirayama: contributed to the research and investigation by specifically performing experiments and data collection; also contributed to the preparation and writing of the original draft. Sae Namba, Yuriko Hoshino and Takachika Hoshino: performed experiments and assisted in writing the original draft. Kazuharu Yamato: contributed to planning and leading experiments on vinegar production and fermentation. Munehiro Hoshino and Mitsuru Sasaki: contributed to the management, leadership, and coordination of the research activity planning and execution, as well as reviewing and editing of the original draft, specifically through critical review, commentary, and revision. All authors have read and approved the final manuscript.
Funding
There was no external funding for this research.
Data availability
Data will be made available upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- Ministry of Agriculture, Forestry and Fisheries. Survey on Recycling of Food Waste. FY2022. Ministry of Agriculture, Forestry and Fisheries (2024).
- Ministry of the Environment. Chapter 3,2 (e). White Paper on the Environment, Sound Material-Cycle Society and Biodiversity. 2024. Edition, Part 2, Chapter 3, Section 1: Current Status of Generation, Cyclical Use and Disposal of Waste (2024).
- Yamato K, Minami K, Hirayama S, et al. Recovery and liquefaction of nitrogen-containing component and minerals from food processing wastes of vinegar using subcritical water. SN Applied Science 2 (2020): 2081.
- Yamato K, Murakami D, Hirayama S, et al. Food-Grade Vinegar Production from the Extract of Sake Lees Obtained by Subcritical Water Treatment. Journal of Food and Nutrition 7 (2021): 1-12.
- Hirayama S, Murakami D, Fukagusa Y, et al. Research on the development of Value-added Vinegar using Subcritical Treated Water, Journal of Food Science and Nutrition Research 6 (2023): 111-116.
- Adachi S. Properties of Subcritical Water and Its Utilization. Chemistry and Biology 47 (2009): 697-702.
- Goto M. Properties of Sub- and Supercritical Water and its Application. Journal of Smart Processing 6 (2017): 202-205.
- Gaecia-Mario M, Rivas-Gonalo JC, Ibanez E, et al. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal.Chim.Acta 563 (2006): 44-50.
- Yoshida H, Terashima M, Takahashi Y. Production of organic acids and amino acids from fish meat by sub-critical water hydrolysis. Biotechnol. Prog 15 (1999): 1090-1094.
- Doumoto A, Hara M, Yoshida M. Fujiwara S. Development of Strawberry Soil Using Hydrothermal Decomposition of Food Waste. Japanese Journal of Soil and Fertilizer Science 90 (2019): 153-157.
- Miyashita T, Yokota T, Kido K, et al. Improvement of the Bitterness and Astringency of Green Tea by Sub-Critical Water Extraction. Food Science and Technology Research 19 (2013): 471-478.
- Ishak H, Yoshida H, Noor Azura Muda, et al. Rapid Processing of Abandoned Oil Palm Trunks into Sugars and Organic Acids by Sub-Critical Water 7 (2019): 593.
- Wakita Y, Harada O, Kuwata M, et al. Sub-and Supercritical Water Treatment of Sake (Japanese Rice Wine) Cake and Physiological Functions of Soluble Components. J.Brew.Soc.Japan 100 (2005): 49-55.
- Marshall W, Franck E. Ion product of water substance, 0-1000°C, 1-10000 bars - new international formulation and its Background. J Phys Chem 10 (1981): 295-304.
- Arakawa Y, Akagi II, Yamamoto K. Determination of ammonium nitrogen in KCl extracts of cropland soils by using 2-hydroxybiphenyl sodium salt. J Soc Soil Sci Plant Nut. 74 (2003): 657-659.
- Ran Y, Wang YZ, Liao Q, et al. Effects of operation conditions on enzymatic hydrolysis of high-solid rice straw. International Journal of Hydrogen Energy 37 (2012): 13660-13666.
- Liu FZ, Yamada I, Mori H, et al. A Method of Calculating Multistage Liquid-Liquid Extraction for Ternary Systems. Journal of Chemical Engineering of Japan 24 (1991): 261-264.
- Kubo A. Degradation Methods of Biological Samples for Inorganic Analysis - Wet Oxidation and Focusing on dry incineration. Bunseki Kagaku 11 (1962): 864-871.
- Japanese Industrial Standards. Sodium carbonate (2019).
- Report of the Subdivision on Resources the Council for Science and technology Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard tables of food composition in Japan (Eighth Revised Edition) (2023).
- Nojiro K, Kozaki M, Yosii H, et al. Brewing Science, Kodansha, Inc. 2010: 206-207.
- Japanese Agricultural Standards “Brewed Vinegar” (2020).
- Nanba T, Kato H. Changes in Several Components during Acetic Acid Fermentation,Nippon Shokuhin Kogyo Gakkaishi. 1985;32(9): 646-654.
- Josiel MC, Monique MS, Marleny DA, et al. Recent Advances in the Processing of Agri-food By-products by Subcritical Water. Food and Bioprocess Technology16 (2023): 2705-2724.
- William GS, Juliane V, Luiz ENC, et al. Recovery of sugars and amino acids from brewers' spent grains using subcritical water hydrolysis in a single and two sequential semi-continuous flow-through reactors. Food Research International 157 (2022): 111470.
- Gisselle RA, Irina K, Silvana CL, et al. Health-promoting peptides in fermented beverages. Revista Argentina de Microbiología 56 (2024): 336-345.
- Yonghui Yu, Goutom KG, Linyue Z, et al. The classical and potential novel healthy functions of rice bran protein and its hydrolysates. Critical Reviews in Food Science and Nutrition 62 (2021): 8454-8466.
- Tanase R, Senda R, Matsunaga U, et al. Taste Characteristics of Various Amino Acid Derivatives. Journal of Nutritional Science and Vitaminology 68 (2022): 475-480.
- Shimadzu Corporation Amino Acid Analysis System (2025).
- Shimadzu Corporation Elemental Analysis Base Paper Spectrophotometer (2025).
- SHIMADZU Corporation "TOC Measurement Methods: TOC Academy" (2025).